Biology Reference
In-Depth Information
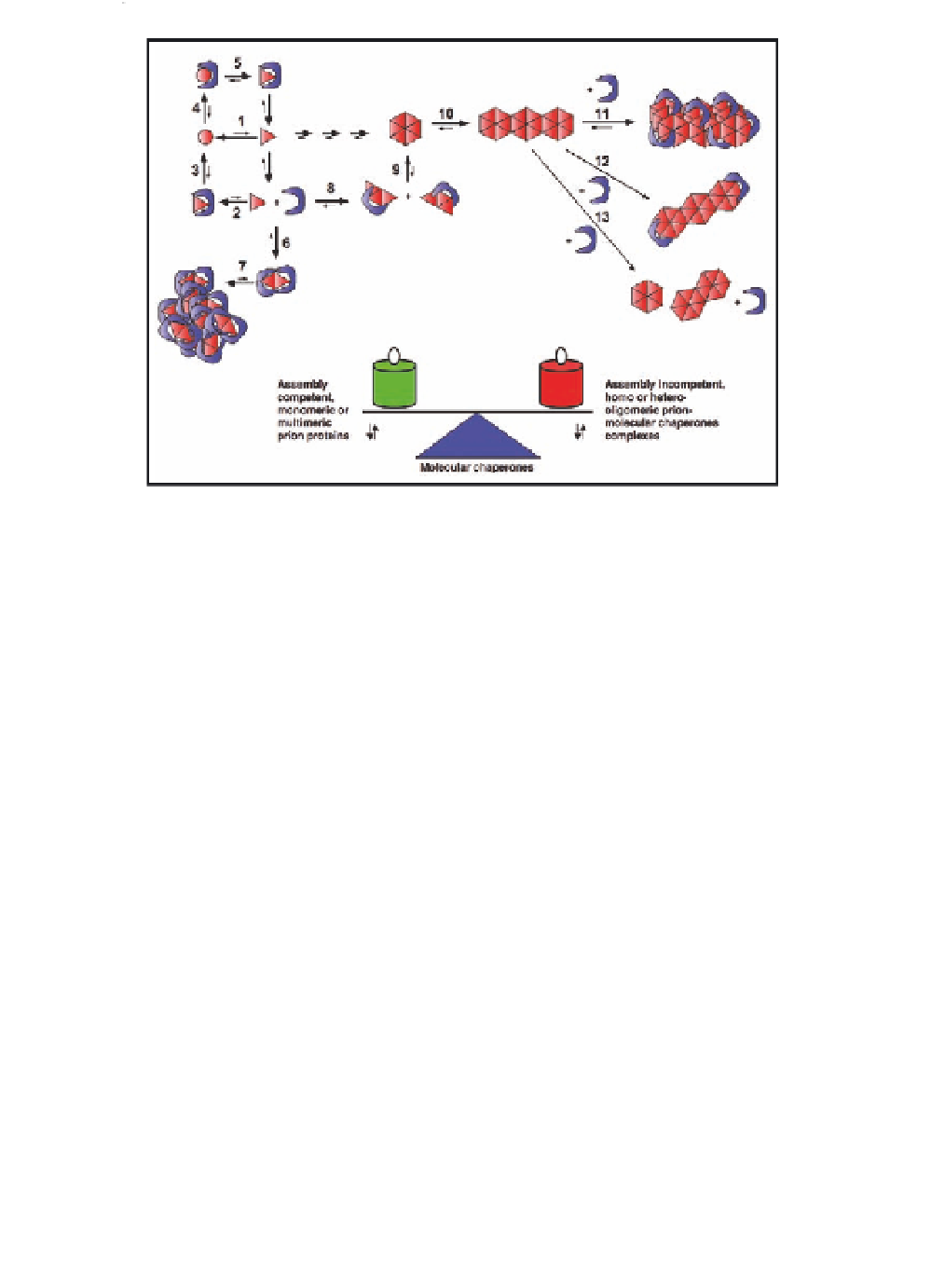
Figure 6.5
Modulation of prion aggregation by molecular chaperones.
Molecular chaperones (in blue) can interact either with the
abnormal form of the prion protein (red triangle, reaction 2)
generated by a folding event (reaction 1), or the constitutive
form of the prion (red circle, reaction 4). The interaction of
molecular chaperones with the abnormal form of the prion
can lead to the refolding of this form into the constitutive form
(reaction 3). The interaction of molecular chaperones with the
constitutive prion can lead to the generation of an abnormal
form of the prion (reaction 5). Following the interaction of
molecular chaperones with the abnormal, assembly competent
form of the prion, an assembly incompetent low (reaction 6) or
high (reaction 7) molecular weight complex can be generated.
Molecular chaperones can also interact with an assembly
competent oligomeric form of prions (reaction 8) and favour
stable nuclei formation (reaction 9). Finally, molecular
chaperones can interact with the walls of fibrillar prions and
lead to the formation of fibrillar bundles (reaction 11) or the
capping of fibril ends (reaction 12), or they can fragment the
fibrils (reaction 13). Reactions 4, 5, 8, 9, and 13 favour prion
assembly, while reactions 2, 3, 6, 7, 11 disfavour assembly.
Thus, the functional differences between molecular chaperone
actions can modulate the propagation of prion traits through
a fine tuning of the oligomeric state of prion proteins. See also
Colour Insert.
Four factors contribute to the extreme difficulty in assessing
the role of molecular chaperones from observations made
in vivo.
Search WWH ::

Custom Search