Biology Reference
In-Depth Information
as filamentous actin (Fig. 6.2). Indeed, Sup35p and Ure2p fibrils
are unbranched, approximately 20 nm wide and more than 1 µm
long,
31,43-44
and have increased resistance to proteolysis when
compared to the soluble proteins.
Similar to conventional
amyloids, fibrillar Sup35p and Ure2p bind the dyes Congo red and
thioflavin T, and exhibit yellow-green birefringence in polarized
light upon Congo red binding.
31,43
43,47
These characteristics, together
with the findings that the N-terminal domains of Sup35p and Ure2p
that are critical for prion propagation, assemble
into fibrils
that exhibit a 4.7 Å reflection in X-fibre diffraction images,
in vitro
48,49
led to
the view that full-length Sup35p and Ure2p fibrils are conventional
amyloids. This view needs to be tempered based on the observations
summarized below.
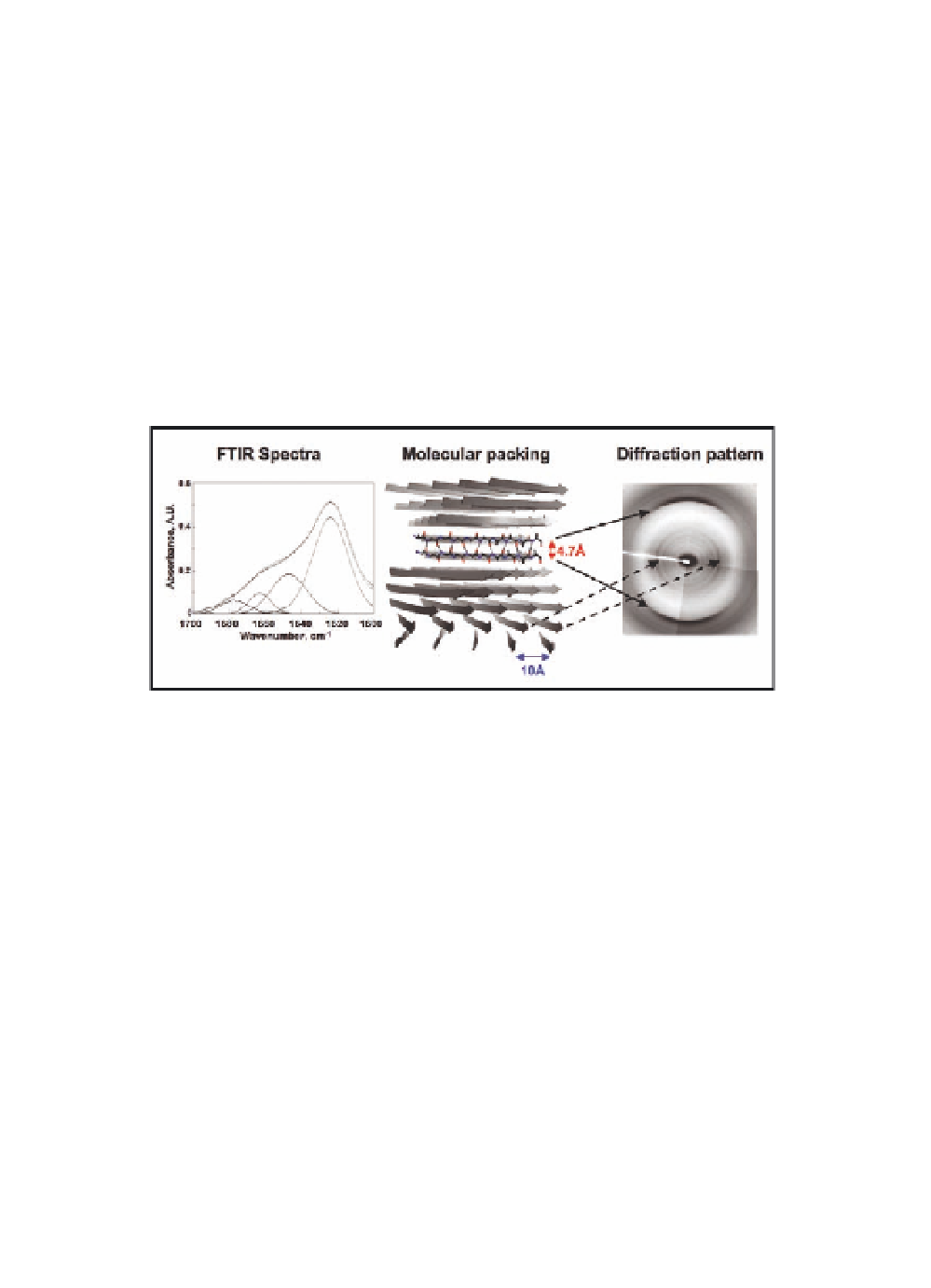
Figure 6.3
Amyloid fibrils structural characteristics. FTIR spectrum of
amyloid fibrils (left panel) showing the amide I absorbance
maximum at 1610-1630 cm
−1
. The
β
-sheet component of the
fibrils absorbs specifically within this wavelength range unlike
the sheet content of soluble proteins. Packing of the
β
-strands
within an amyloid fibril (middle panel). The systematically
H-bonded (dashed lines)
β
-sheet core is represented. Oxygen
atoms are in red while nitrogen atoms are in blue. X-ray
diffraction pattern of amyloid fibrils (right panel). As indicated
with the arrows, the 4.7 Å and 10 Å reflections originate from
the interstrand and intersheet distances, respectively. The
orientation of the strands and sheets relative to the fibril main
axis that is perpendicular to the scheme is shown. See also
Colour Insert.
It is worth recapping first the structural definition of amyloid
fibrils. The term amyloid fibrils refers to fibrillar protein deposits
associated with disease that appear unbranched; that bind the dyes
thioflavin T or S and Congo red, with a typical and concomitant

Search WWH ::

Custom Search