Geology Reference
In-Depth Information
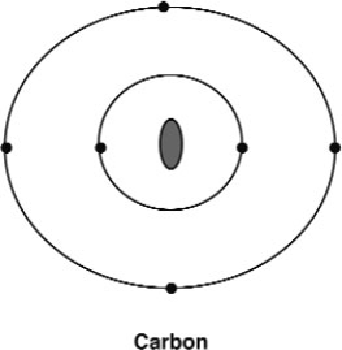
Figure 16: A carbon atom with its four outer electrons.
In order to understand the ways in which the elements contribute to Gaia we need to
learn a further rule of atomic social etiquette, namely that rather than sharing electrons
in a covalent bond, it is possible for an atom to give away one or more outer electrons
if there is an atom available that can use them to complete its own outer orbit. This type
of atomic union is known to chemists as an
ionic
bond, and it happens when the donor
atom tends to have less than four outer electrons. On the other hand, says the rule, if an
atom has more than four outer electrons, it will tend to accept additional electrons from
other atoms when bonding ionically. A good example is common salt, sodium chloride.
The sodium atom has a single outer electron, which it happily gives to a chloride atom
that needs only one electron to complete its outer orbit. By exchanging the electron both
partners find rest, but now the attractions between positive and negative charges come
into play. Having lost an electron, the sodium atom now has one proton in its nucleus
without an equivalent negative charge to balance it, so the sodium atom has become a
being that attracts negative charges. Meanwhile, the chlorine atom, in taking up the elec-
tron, has become a chloride ion and now has an overall negative charge. The sodium
and chloride, as charged atoms (which chemists call
ions
), experience a new restless-
ness—they must lie close to each other so that their charges can interact, an attraction
which manifests, under the right conditions, as the crystals familiar to us as table salt.