Biology Reference
In-Depth Information
IAA
IAA
19C07
CLE
Annexin
10A06
Cell wall degrading enzyme
Chorismate mutase
SPRYSEC19
Nuclear-targeted effectors
Cellulose-binding protein
16D10
Ubiquitin
Auxin transport
Auxin block
PIN1
PIN1
PME3
?
Cleavage
LAX3
19C07
AUX1
cell surface
(secreted)
16D10
SCR-like
alternative
secretory
pathway
CBP
CLE
peptides
?
nuclear
targeting
?
Protein
Degradation
Transcriptional
Reprogramming
PIN3
CLV2/CRN
Developmental
Reprogramming
LAX3
Feeding
tube
Chrorismate
mutase
PIN3
Chrorismate
LAX3
Metabolic
Reprogramming
Annexin
Vacuole
LAX3
Gland
ampulla
Oxidoreductase
10A06
PIN3
PIN3
SPRYSEC19
Stress & Defense
Response
SPDS2
CWDEs
CC-NB-LRR
Current Opinion in plant Biology
Fig. 7.3.
Molecular mechanism of nematode effector protein action in host plant cells (adapted from Gheysen and Mitchum
2011). For a color version of this figure, please refer to the color plate.
origin were involved in this process is still
unclear (Dropkin 1963; Jones 1981). The first
characterized effectors are secreted from a cyst
nematode esophageal encode EGases (Smant
et al. 1998). EGases degrade polysaccharides
possessing
expressed in later parasitic stages, during which
syncytium continues to expand along the vascu-
lature, suggesting cell wall-degrading enzymes
of plant origin play a role in that process. Based
on the study of five tobacco EGases that are
upregulated upon infection by both root-knot and
cyst nematodes, Goellner et al. (2000) proved
that cell wall modification within plant-parasitic-
nematode feeding cells arises from cell-wall-
modifying enzymes of plant, rather than being of
nematode origin (Goellner et al. 2000). Pectate
lyase and polygalacturonase, two other cell-wall-
degrading enzymes, have also been identified
from RKN (Doyle and Lambert 2002; Jaubert
et al. 2002; Popeijus et al. 2000). They likely
play a role in nematode movement by soften-
ing the cell wall. An expansin gene was cloned
from the plant-parasitic roundworm species
Glo-
bodera rostochiensis
(Qin et al. 2004). Expansin
proteins have so far been identified only in plants,
-1, 4-glucan backbones, such as
cellulose and xyloglucan. In their study, the
authors isolated EGase that was synthesized in
the esophageal glands of the cyst nematodes
Glo-
bodera rostochiensis
and
Heterodera glycines
(Smant et al. 1998). The homologous genes were
also identified in RKN and in other cyst nema-
tode species (Dautova et al. 2001; De Meut-
ter et al. 2001). EGase of nematode origin was
shown to be expressed primarily in J2 stage
and again in adult males that migrate out of the
root, supporting a role for it in cell wall degra-
dation during the penetration and intracellular
migration of the nematode through root tissue
(Goellner et al. 2000). Nematode EGases are not
β



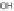



Search WWH ::

Custom Search