Biomedical Engineering Reference
In-Depth Information
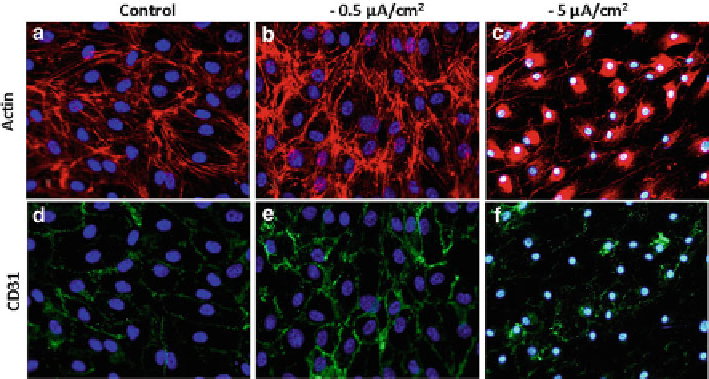
Fig. 4.8
F-actin (
a-c
,
red
) and CD31 (
d-f
,
green
) staining of untreated HDMEC (
a
,
d
) on Ti6Al4V
and after cathodic polarisation of Ti6Al4V alloy for 24 h with −0.5 m A/cm
2
(
b
,
e
) and −5 m A/cm
2
(
c
,
f
, fluorescent microscopy, nuclei were stained blue with Hoechst 33342, scale bar: 50 m m )
This was also true for cathodic polarisation-induced rearrangements of actin
cytoskeleton and loss of intercellular contacts (Fig.
4.8
), this being important for
the regulation of endothelial permeability during inflammation, at the higher cur-
rent densities as well as for cell nuclear condensation. Thus, the cells subjected to
the products of cathodic half-reaction lost typical peripheral actin ring, as well as
intercellular contacts (CD31 staining). This indicated that cathodic half-reaction of
corrosion could induce changes in viability and induce a pro-inflammatory
phenotype of endothelial cells, thus potentially exerting negative effects on wound
healing and stability of an implant.
It was also shown that cathodic polarisation of Ti6Al4V alloy induced drastic
elevation in fluorescence of DCF that was given to the cells prior to the treatment
(Fig.
4.9
). This can result from DCDHF oxidation by H
2
O
2
formed during the
cathodic partial reaction and freely diffusing through the cell membrane or by ROS
induced in the cells by H
2
O
2
.
Apart from H
2
O
2
and ROS formation cathodic half-reaction of corrosion may
result in few additional events. First of all hydroxyl ions are formed in oxygen
reduction reactions, which might lead to the change in pH. However, the experi-
ments were performed in a buffered system ruling out the possibility of a pH
effect. Secondly, oxygen deficiency may occur locally due to oxygen consump-
tion in the cathodic half-reaction. In fact, decreased oxygen concentrations were
detected in close proximity to the surface of polarised titanium [
24
] . This effect
correlated with the reduced spreading of osteoblasts on polarised titanium.
However, the authors did not take into consideration other events occurring during
cathodic half-reactions. Finally, hydrogen might be formed as a result of the
cathodic half-reaction. However, this reaction is prevalent at acidic pH or when
Search WWH ::

Custom Search