Biomedical Engineering Reference
In-Depth Information
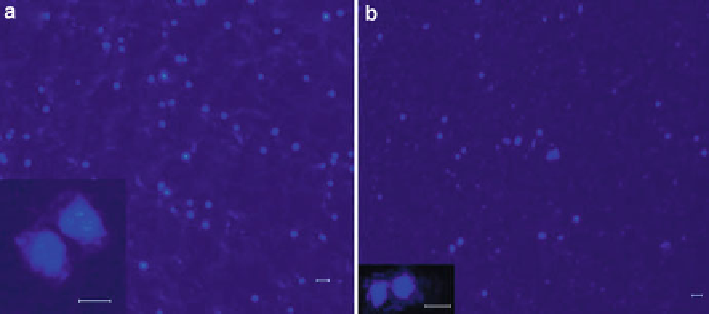
Fig. 8.9
Fluorescently stained cells on Ti substrates. (
a
) HRN-coated Ti. (
b
) Uncoated Ti
(magnification: 20×; inset magnification 200×). Bars = 60 m m,
inset
bars = 50 and 100 m m for
(
a
) and (
b
), respectively [
15
]
hydroxyapatite; this was measured as a function of the cell adhesion of human fetal
osteoblast (hFoB) cells (Fig.
8.9
).
Webster et al. [
96
] incorporated carbon nanofibres (CNF) into polycarbonate-
urethane for neural or orthopaedic prosthetic devices. They reported that this mate-
rial had the potential to increase neural and osteoblast functions, as cell attachment
increased with CNF loading. Additionally they stated that the functions of cells that
contributed to glial scar-tissue formation for neural prostheses (astrocytes) and
fibrous-tissue encapsulation for bone implants (fibroblasts) decreased on the poly-
carbonate-urethane composites containing increasing amounts of CNF. In this man-
ner, this study provided the first evidence that CNF formulations may interact with
neural and bone cells, which is important for the design of successful neural probes
and orthopaedic implants.
Furthermore, Webster et al. [
96
] summarised that using nanotechnology in bio-
logical systems may be potentially feasible as biological systems are governed by
molecular behaviour at the nanoscale, and therefore the properties of which are
accustomed to high levels of interaction at this nanoscale. This study by Webster
et al. highlighted the potential for CNT to be used in PMMA bone cement to
encourage cell growth at the bone-cement interface with the aim of reducing
aseptic loosening by enhancing the mechanical interlock in the cancellous bone.
As mentioned previously, CNTs exhibit many unique mechanical, thermal and
electrical properties. However, their potential use for biomaterials or biomedical
applications is almost wholly dependent on their biocompatibility. Cui et al. [
17
]
investigated the effect of SWCNT on human HEK293 cells (human embryo kid-
ney cells). Results showed that SWCNT could inhibit HEK293 cell proliferation,
inducing cell apoptosis (programmed cell death as controlled by the nuclei in
normally functioning cells) and decreasing cellular adhesive ability in a dose- and
time-dependent manner. Their results also showed that HEK293 cells initiated
Search WWH ::

Custom Search