Biomedical Engineering Reference
In-Depth Information
O
H
3
C
CH
3
O
CH
3
O
O
Catalyst
OCH
CO
CH
C
O
Heat
CH
3
n
O
Lactide
Polylactic acid
Fig. 6.1
The polymerization of lactide to polylactic acid
In general, polymer degradation is accelerated by an increased hydrophilic
tendency of the polymer backbone or in the extremities, increased reactivity among
hydrolytic groups in polymer chain, a low crystallinity, more pronounced porosity
(larger pore sizes), and lower ends.
A polymer is generally named based on the monomer it is synthesized from. For
both lactic acid and glycolic acid, an intermediate cyclic dimer is prepared and purified,
prior to polymerization. These dimers are called lactide and glycolide, respectively.
PLA is an aliphatic polyester, which can be easily produced through ring-opening
polymerization of lactide using a stannous octoate catalyst (Fig.
6.1
).
PLA has chiral carbon in its structure and thus can exist as two stereoisomers,
called poly-L-lactic acid (PLLA) and poly-D-lactic acid (PDLA), or as a racemic
mixture called poly-DL-lactic acid (PDLLA). Most current interference screws are
from PLLA, a polymer with semicrystalline structure, a glass transition temperature
between 55 and 60°C, and a melting temperature between 173 and 178°C [
22
]
which has good strength and long degradation period (2-5 years). Instead poly-DL -
lactide has an amorphous structure with a lower strength and shorter degradation
period (12-16 months) making it a very interesting material for drug delivery appli-
cation. Copolymers of PLLA with PDLA or PDLLA have also been studied because
when adding d-isomers the degradation rate changes as a consequence of the
increase of amorphous regions and decrease of crystallinity. The properties of PLLA
and its copolymers can be affected by factors related to material, such as polymer-
ization method, L/D ratio, and thus molecular weight, polydispersity, degree of
chain orientation, and by factors related to implant like porosity, size, and shape of
the implant [
23
] .
The PGA is a stronger acid, and highly crystalline (around 50%) with a glass
transition temperature between 35 and 40°C and melting point of 225-230°C [
24
] .
The ring-opening polymerization (Fig.
6.2
) of the cyclic diester of glycolic acid
allows the obtaining of polymers with high molecular weight, containing 1-3%
residual monomer. According to the data from scientific literature it fully degrades
in 3-6 months [
25,
26
] .
PGA is more hydrophilic and thus more susceptible to hydrolysis than PLA;
even the screws made by PGA show similar results regarding the clinical results,
with a tendency to faster degradation. A clinical review of these models concludes
that the rate of polymer degradation can be related to the size, geometry, and, finally,
the design of the implant [
25
] .


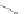




















Search WWH ::

Custom Search