Biology Reference
In-Depth Information
to be rapidly inactivated. In general, two mechanisms are known that could remove
endocannabinoids from the synaptic cleft to ensure rapid signal inactivation: re-uptake or
enzymatic degradation. AEA is inactivated by reuptake [22, 24] via uncharacterized
membrane transport molecule, the 'AEA membrane transporter' (AMT) [20, 22, 23, 75, 93,
94, 124], and subsequent intracellular enzymatic degradation. AEA is metabolized to
arachidonic acid and ethanolamine via the action of the fatty acid amide hydrolase (FAAH),
and this activity plays a significant role in the rapid clearance of AEA from extracellular
compartments [56, 77]. In addition to hydrolysis by FAAH, AEA is metabolized by COX-2,
LOX and cytochrome P450. COX-2 has been shown to metabolize AEA in to PGE
2
-
ethanolamide (PGE2-EA) [173]. 12- and 15-LOX, nonheme iron-containing enzymes convert
AEA into 12- and 15-hydroxy-AEA (12- and 15-HAEA) in vitro, respectively [115, 124].
Cytochrome P450 also metabolizes AEA into several polar lipids [32]. Recently, in the
absence of FAAH, exogenously injected AEA has been shown to be converted into o-
phosphorylcholine (PC)-AEA in the brain and spinal cord. The choline-specific
phosphodiesterase (NPP6) was found to convert PC-NAE into NAE [150]. Further research is
required to elucidate the exact mechanism and enzymes involved in this pathway of AEA
metabolism.
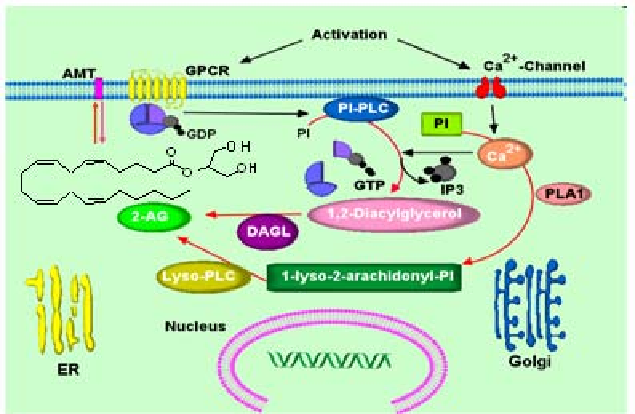
Figure 3. The potential pathways of 2-arachidonylglycerol biosynthesis. Intracellular Ca2+ initiates 2-
AG biosynthesis by inducing the formation of diacylglycerol (DAG) in the membrane by stimulating
the phosphatidyl-inositol-phospholipase C (PI-PLC) pathway. 2-AG is the product of DAG-lipase
(DAGL) acting on DAG. The second pathway involves hydrolysis of PI by phospholipase A1 (PLA1)
and hydrolysis of the resultant lyso-PI by a specific lyso-PLC. Once synthesized, 2-AG can transported
to the outside of the cell through a process that has not yet been characterized. AMT, anandamide
membrane transporter
The second widely recognized endogenous CB1 agonist is 2-arachidonylglycerol (2-AG)
was characterized soon after the discovery of AEA [141, 187]. 2-AG has been characterized
as a unique molecular species of monoacylglycerol isolated from both the canine gut [141]
and the rat brain [188], where it presumably functions as an endogenous cannabinoid receptor
ligand. 2-AG biosynthesis occurs by two possible routes in neurons, which are illustrated in