Environmental Engineering Reference
In-Depth Information
ii. Particle diffusion mechanism, in which transport of adsor-
bate within the pores of the adsorbent takes place except for
a small amount of adsorption, which occurs on the external
surface.
iii. Adsorption of adsorbate on the interior surface of the
adsorbent.
Out of the above three processes the third one is very rapid and does not
represent the rate-limiting step in the uptake of organic compounds [89].
While the other two steps give rise to the following three distinct cases:
Case I :
External Transport > Internal Transport
(Here rate is governed by particle diffusion)
Case II :
External Transport < Internal Transport
(Here rate is governed by film diffusion)
Case III:
External Transport ≈ Internal Transport
In Case III the transport of ions to the boundary may not be possible at
a considerable rate, thereby leading to the formation of a liquid film with a
concentration gradient surrounding the sorbent particles.
In accordance to Fick's first law the flux J (in moles per unit time and
unit cross-section, normal to the path of flow) of the diffusing ion [89] is
given by equation:
DC
R
(11.15)
J
i
where C is concentration (in moles per unit volume), 'Di'
i
' is effective
diffusion coefficient and 'r' gives the direction along which transport is
taking place.
Using Fick's second law, the governing differential equation used for a
spherical exchanger bead of radius r
o
in a solution can be written as:
C
t
2
C
r
C
rC
(11.16)
D
2
i
2
Using the proper boundary conditions, the solution of this equation has
been obtained as an infinite series, i.e.,
Q
Q
(11.17)
F
t






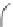







Search WWH ::

Custom Search