Environmental Engineering Reference
In-Depth Information
contact time study is helpful in determining the duration of equilibrium
attainment, i.e., the time beyond which no further significant adsorption
takes place over the surface of adsorbent. Thus, a contact time study is very
much helpful in calculating the kinetic data for the rate-determining step.
Under the contact time studies, uptake capacities of the dyes are determined
by taking a fixed concentration of the dye and varying the amount of the
Hen Feather at different temperatures. When the equilibrium is thought to
have been established, the solutions are filtered after definite time intervals
and noted for the change in the absorbance. Thus, contact time studies
are helpful in determining the uptake capacities of Tartrazine-Hen Feather
and Amaranth-Hen Feather systems at 30, 40 and 50 C temperatures.
11.4.2
Results and Discussions
11.4.2.1
Effect of pH
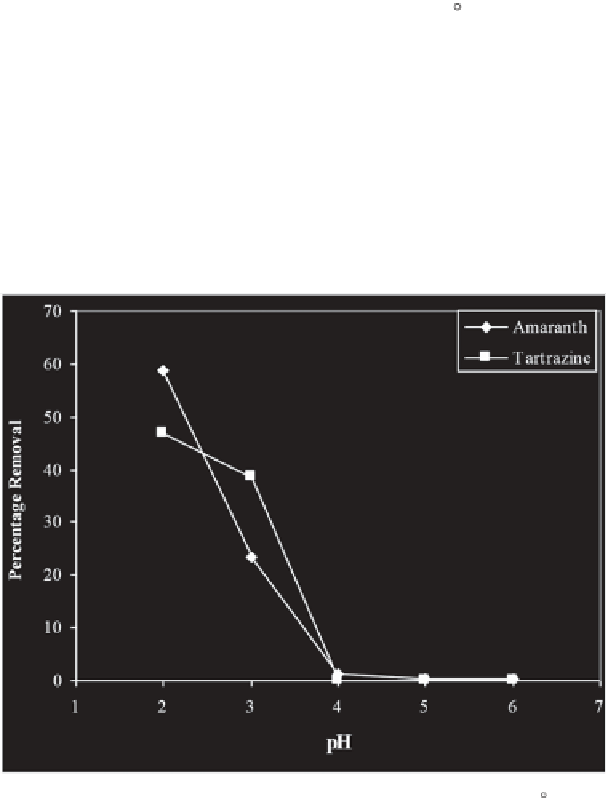
Figure 11.1 presents variations of the adsorption of undertaken Azo dyes
by Hen Feather over a wide range of pH in terms of percentage of the dye
adsorbed. The results indicate that the extent of adsorption of each dye is
Figure 11.1
Effect of pH on adsorption of Azo dyes over Hen Feathers at 30 C.
(Amaranth: Dye Concentration = 10 × 10
-5
M, Adsorbent Dose = 0.01 g/25mL;
Tartrazine: Dye Concentration = 9 × 10
-5
M, Adsorbent Dose = 0.01 g/25mL).
Search WWH ::

Custom Search