Environmental Engineering Reference
In-Depth Information
even chemical degradation [27,28]. This renders azo dyes highly nonde-
gradable compounds [29]. Azo dyes, their precursors and biotransforma-
tion products such as aromatic amines are toxic [30], carcinogenic [31,32]
and mutagenic in nature [33]. Thus, the removal of azo dyes from waste-
water becomes highly important.
In the present case, two azo dyes, Tartrazine and Amaranth, have been
considered for the removal process. Their important physicochemical
properties like molecular weight, physical state, color, melting point, odor,
solubility in water, specific gravity and absorption maximum, etc., are pre-
sented in Tables 11.1 and 11.2.
11.2.1 Tartrazine
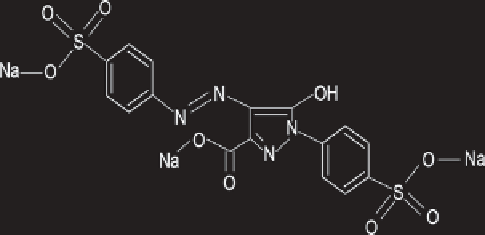
Scheme 11.1
Chemical structure of Azo Dye-Tartrazine.
Tartrazine (C
16
H
9
N
4
Na
3
O
9
S
2
, molecular weight 534.4; Scheme 11.1),
IUPAC name Trisodium-5-hydroxy-1-(4-sulfonatophenyl)-4-(4-sulfona-
tophenylazo)-H- pyrazole-3-carboxylate is a coal tar dye belonging to the
azo class of dyes (Table 11.1). It has a light orange color and being that it
is the least expensive synthetic color it is widely used for the coloring of
wool, silk and other textile materials, cosmetics [34] and pharmaceuticals
[35,36]. The presence of polar groupings renders it highly water soluble.
It is used as food additive in a variety of food materials [37-40]. It is also
used extensively in laboratories as either biological stains or pH indica-
tors. The wide applicability of the dye, especially in foodstuffs, has initiated
global interest regarding its toxicological impact on living systems and the
environment.
Various review articles have appeared in the literature describing
Tartrazine as an initiator of allergies, asthma [41,42], and dermal diseases
in humans [43,44], and corneal staining of the eyes [45]. Many publications
have appeared highlighting the effects of Tartrazine on the lungs, leading
Search WWH ::

Custom Search