Environmental Engineering Reference
In-Depth Information
represented by the Equations 10.1 and 10.2, where x is the amount of dye
adsorbed (mg); m is the amount of adsorbent used (g); C
e
(mg L
-1
) and q
e
(mg g
-1
) are the liquid phase concentration and solid phase concentration
of adsorbate at equilibrium, respectively; K
L
(L g
-1
) and a
L
(L mg
-1
) are the
Langmuir isotherm constants. The Langmuir isotherm constants, K
L
and
a
L
are evaluated through linearization of Equation 10.1. Hence by plotting
C
e
/q
e
against C
e
it is possible to obtain the value of K
L
from the intercept,
which is 1/K
L
, and the value of a
L
from the slope, which is a
L
/K
L
. he theo-
retical monolayer capacity is q
max
and is numerically equal to K
L
/a
L
.
x
m
KC
aC
q
Le
(10.1)
e
1
Le
C
qK
1
a
K
(10.2)
e
L
C
e
e
L
L
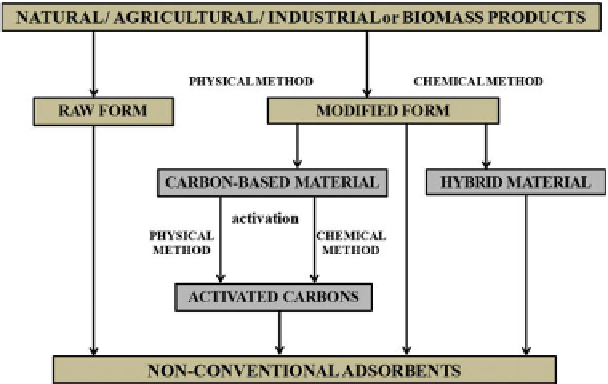
The main non-conventional adsorbents studied in the literature and
employed as non-conventional adsorbent for dye removal include acti-
vated carbons (AC) from byproducts or solid wastes, clays and clay-based
materials, zeolites, agricultural wastes (including byproducts from for-
est industries), industrial byproducts, biosorbents such as peat, chitin/
chitosan and biomass, polysaccharides such as starch-based materials and
alginates, and other miscellaneous materials such as cotton or substances
capable of forming host-guest complexes such as cyclodextrins and calix-
arenes (Figure 10.1). In this chapter, an extensive list of non-conventional
adsorbent literature has been compiled. Results in terms of adsorption
Figure 10.1
Schematic representation of non-conventional adsorbents from solids [8].




Search WWH ::

Custom Search