Environmental Engineering Reference
In-Depth Information
from 2.0 to 9.0, and then remained almost constant. Similar results were
observed by Al-Khatib
et al.
[29] for methylene blue uptake on bentonite.
A contrary result by Eren and Afsin [27] showed that cationic Crystal
Violet adsorption on natural and Ni-, Co-, Zn-pretreated bentonite is
pH independent. They explained that adsorption occurs partly by ion
exchange in the interlayer and on basal plane surfaces, and partly via non-
coloumbic interactions between an adsorbed cation and a neutralized site.
Turabik [30] also found that bentonite adsorption of Basic Red 46 and
Basic Yellow 28 basic dyes are pH independent in the range of pH 2.0-8.0.
Kurniawan
et al.
[49] prepared rarasaponin-bentonite to adsorb basic
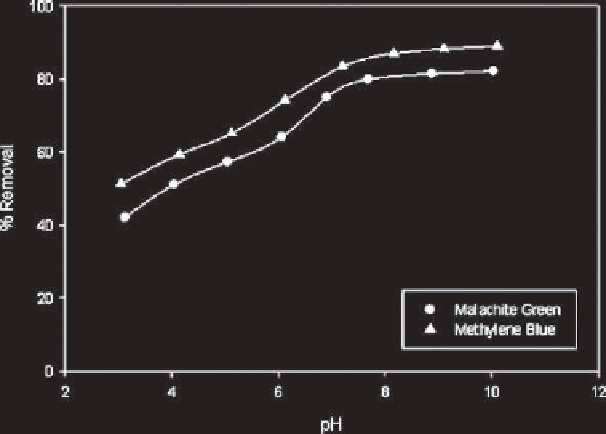
methylene blue (MB) and malachite green (MG). They found that dye
removal gradually increases in the pH range of 4-7 and reaches its maxi-
mum at around pH 8 (Figure 9.2). They described a competitive adsorption
mechanism between hydrogen ions (H
+
) and dye molecules. Moreover, a
high concentration of H
+
ions causes protonation of silanol groups that
repels cationic dye molecules. As the pH of the system increases, the con-
centration of H
+
ions decreases and the silanol sites become deprotonated,
in turn increasing the negative charge density on the adsorbent surface and
facilitating the adsorption of positive dye molecules. Even though uptake
of pollutants generally increases with increasing surface area, Kurniawan
et al.
[49] also showed that in this case specific surface area has no effect on
the adsorption. The surface area of bentonite decreases with these added
surfactants (51.8 m
2
/g decreases to 45.3 m
2
/g), but dye recovery increases,
Figure 9.2
Variation of pH with adsorption of dyes onto rarasaponin-bentonite [49].
Search WWH ::

Custom Search