Environmental Engineering Reference
In-Depth Information
homogeneous or in heterogeneous systems due to its versatility. The
Redlich-Peterson isotherm is given by Equation 8.3 [122]:
kC
1aC
q
RP
e
(8.3)
e
RP
e
where k
RP
and a
RP
are the Redlich-Peterson constants (L g
-1
) and (L mg
-1
)
β
,
respectively, and β is the heterogeneity coefficient, which varies between 0
and 1. The Redlich-Peterson model was adequate to represent the bio-
sorption of FD&C Red 40 onto chitosan [59] and food dyes onto chitosan
films [69].
Another model usually employed to represent the biosorption pro-
cesses is the Dubinin-Radushkevich model [123], as demonstrated in
Equation 8.4:
qq (
B)
2
(8.4)
e
S
where q
s
is the D-R constant (mg g
-1
) and ε can be related as Equation 8.5:
1
C
(8.5)
RTln(1
)
e
where R is the universal gas constant (8.314 J mol
−1
K
−1
) and T the tem-
perature (K). The B constant is relative to the biosorption free energy E (kJ
mol
-1
), as demonstrated in Equation 8.6 [123]:
1
2B
(8.6)
E
The Dubinin-Radushkevich model was adequate to describe the bio-
sorption of Basic Blue 9 onto nut shells and Eichhornia [128].
The Sips isotherm is a combination of the Langmuir and Freundlich
isotherms, and is given by Equation 8.7 [124]:
m
q(kC)
1+(k C )
(8.7)
q
mS
S
e
e
m
Se
where q
mS
is the maximum biosorption capacity from the Sips model
(mg g
-1
), k
S
is the Sips Constant (L mg
-1
)and m the fractionary exponent
related with the biosorption mechanism [124]. The Sips model was ade-
quate to describe the biosorption of food dyes onto
Spirulina platensis
[19],
Reactive Black 5 onto aqai stalks [32] and Remazol Black B onto Brazilian
pine-fruit shells [129].





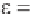







Search WWH ::

Custom Search