Environmental Engineering Reference
In-Depth Information
formation via condensation in mesopores, whereas type V describes a
similar process, but with both strong and weak adsorbate-adsorbent inter-
actions [96,97,112,115,117].
In order to improve the design of a biosorption process, it is important
to establish the more adequate model to represent the equilibrium curve.
There are many mathematical descriptions of biosorption isotherms,
some of which are based on a simplified physical picture of sorption and
desorption, whereas others are purely empirical and intended to corre-
late the experimental data in simple equations with two or more empiri-
cal parameters [116-119]. Among all of these models, the more usual for
the biosorption of SODs are: Langmuir [120], Freundlich [121], Redlich-
Peterson [122], Dubinin-Radushkevich [123], Sips [124] and Tóth [125].
A basic assumption of the Langmuir model is that biosorption takes
place at specific homogeneous sites within the biosorbent, and once a dye
molecule occupies a site, no further biosorption can take place at that site
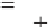
[120]. The Langmuir model is presented in Equation 8.1:
qkC
1kC
(8.1)
q
mL e
e
Le
where q
m
is the maximum biosorption capacity (mg g
-1
) and k
L
is the
Langmuir constant (L mg
-1
). The Langmuir model was found to be adequate
for the following biosorption systems: wood apple shell basic dyes [26],
grapefruit peel-Crystal Violet [27], princess tree leaf-Basic Red 46 [29] and
chitosan-acid dyes [64].
The Freundlich isotherm model is the earliest known relationship
describing the biosorption process. The model applies to biosorption on
heterogeneous surfaces with interaction between adsorbed molecules.
The application of the Freundlich equation also suggests that biosorption
energy exponentially decreases on completion of the sorptional centers of a
biosorbent. This isotherm is an empirical equation which can be employed
to describe heterogeneous systems and is expressed in Equation 8.2 [121]:
(8.2)
1/n
F
qkC
e
F
e
where k
F
is the Freundlich constant ((mg g
-1
)(mg L
-1
)
-1/n
F
) and 1/n
F
the heterogeneity factor. The following biosorption systems were well
described by the Freundlich model: pine cone-Acid Blue 7 [30], rice husk-
Malachite Green [126], palm shell powder-Reactive Blue 21 [127] and
chitosan-Reactive Red 141 [127].
The Redlich-Peterson isotherm is used to represent biosorption equi-
librium over a wide concentration range, and can be applied either in

Search WWH ::

Custom Search