Environmental Engineering Reference
In-Depth Information
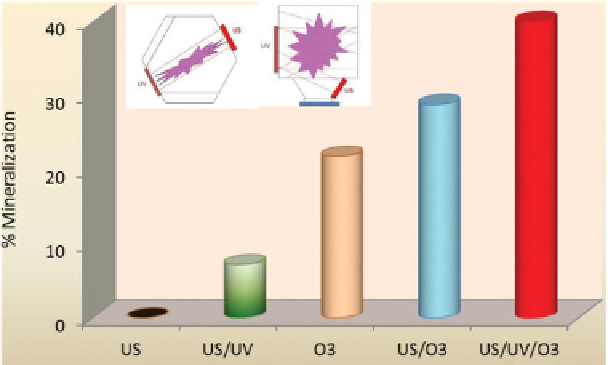
Relative performance of singly applied ultrasound and ozone, and
combinations thereof, for the mineralization of C.I. Acid Orange 7 in
a hexagonal UV-equipped sonoreactor (Figure 7.4h) is presented in
Figure 7.5. The insets on top show the geometry of the reactor and the
position/distribution of UV-irradiation relative to the cavitation clouds.
The efficiencies of each process for bleaching and mineralization of the
dye under the same conditions were as follows: US: 70%, 0%; O
3
: 72%,
24%; US/UV: 84%, 10%; US/UV/O
3
: 100%, 41%. The outstanding syn-
ergy of the trio is the result of enhanced rate of ozone mass transfer and
decomposition (sonolytic and photolytic), excess HO and other reactive
species formation including the singlet oxygen, which is not only more
stable than HO , but also reacts strongly with a wide range of organic
chemicals via hydrogen abstraction [78,79].
7.3.3 Sono-Fenton(US/Fe
2+
) and Sonophoto-Fenton
(US/UV/Fe
2+
)
The sono-Fenton process, which involves sonolysis in the presence of per-
oxide and a ferrous salt to generate ferro-hydroxyl complexes and reactive
oxygen species has received considerable attention for destroying textile
dyestuff and other synthetic dyes [99,100,84,44]. The process is quite sen-
sitive to the operation parameters, specifically to pH, temperature, con-
centrations of ferrous/ferric ions and peroxide, and to the power density.
Figure 7.5
Relative performance of single and combined US, UV, O
3
for the
mineralization of C.I. Acid Orange 7 during 1-h reaction (C
0
= 57 μM, T = 25 C,
I
0
= 2.4x10
-4
Einstein m
-2
s
-1
, 520 kHz, O
3
flow = 0.25 L min
-1
).



Search WWH ::

Custom Search