Environmental Engineering Reference
In-Depth Information
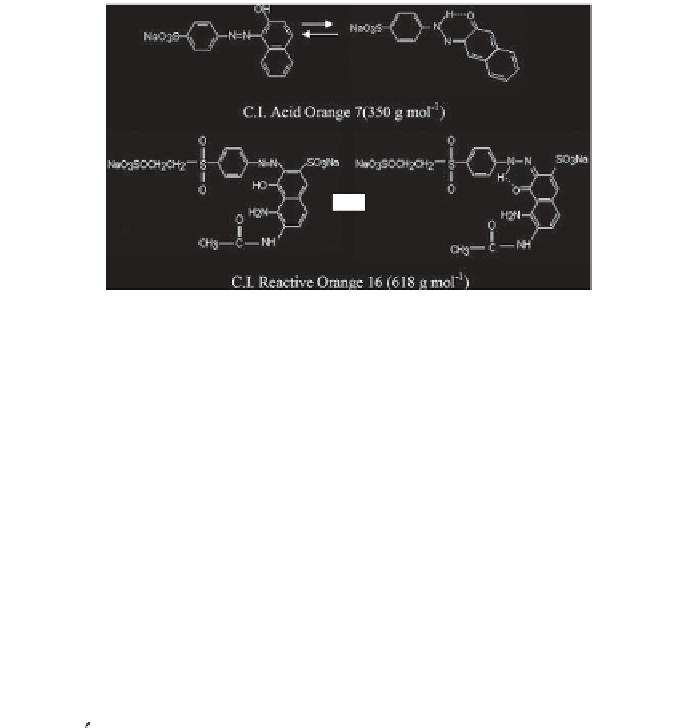
hydrazone
azo
azo
hydrazone
Figure 7.3
Tautomerization of two azo dyes with dissimilar structures.
The rate of azo dye degradation by ultrasound follows second-order
reaction kinetics as r=k[Dye][HO
], where r is the rate of decolorization
and k is the second order reaction rate constant. Accordingly, in many of
the published literature the rate of decolorization has been fit to the simple
pseudo-first-order kinetic model r=k [Dye], where k is the apparent rate
constant as estimated by regression analysis. Some other researchers agree-
ing on pseudo-first-order kinetics have claimed that the rate must follow
a Langmuirian type of adsorption model that considers gas-liquid hetero-
geneity [35,46]:
kKDye
KDye
[
]
(7.14)
0
r
1
[
]
0
where: k and K are the apparent rate and adsorption equilibrium con-
stants, respectively and [Dye]
0
is the initial dye concentration.
7.2.2
Operation Parameters in Decolorization/Degradation
of Textile Dyes by Ultrasound
It has been much reported that the relation between cavitational yield and
the applied power is parabolic, i.e., the yield increases with an increase in
the applied power up to a definite optimum or a threshold, beyond which
it begins declining. The mutual agreement in the literature on reduced effi-
ciency at too high powers is based on the “cushioning” effect, i.e., the bar-
rier exerted by the dense cloud of cavity bubbles to the transfer of acoustic
energy through the liquid [47]. Among a number of mathematical formu-
las that describe the correlation between the efficiency of cavitational yield
and the applied acoustic power, that proposed by Sivakumar
et al.
[24]







Search WWH ::

Custom Search