Environmental Engineering Reference
In-Depth Information
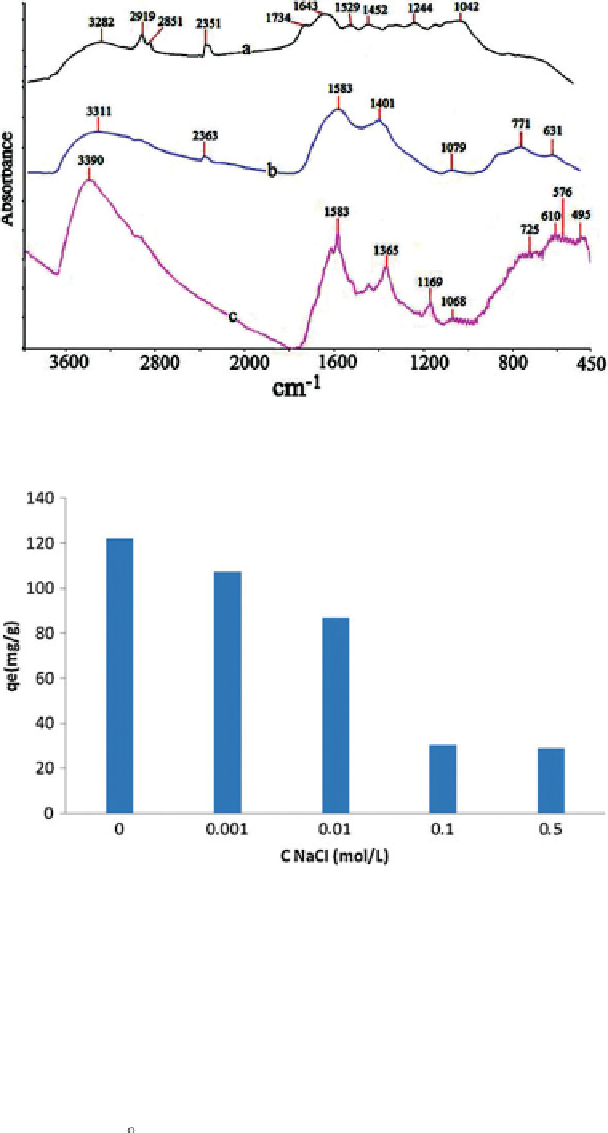
Figure 5.29
FTIR spectra of (a) untreated material, (b) activated carbon and (c) dye-
loaded adsorbent [118].
Figure 5.30
The effect of ionic strength on sorption of malachite green onto the activated
carbon [118].
the adsorbate are oppositely charged at the experimental pH level (pH 3),
the addition of salt has resulted in the reduction of electrostatic interac-
tion between the dye molecules and the activated carbon surface, leading
to a drastic decrease in the adsorption capacity of the adsorbent. A simi-
lar activation method using potassium acetate was employed by Auta and
Hameed [119]. The tea waste was impregnated in potassium acetate and
carbonized at 800 C under purified nitrogen atmosphere. A surface area
Search WWH ::

Custom Search