Environmental Engineering Reference
In-Depth Information
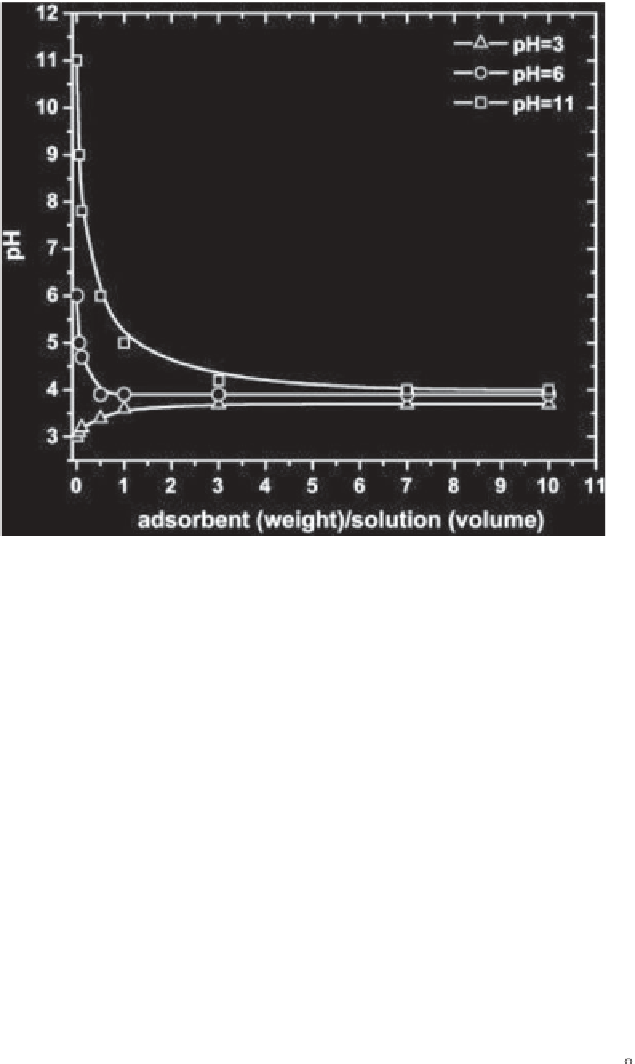
Figure 5.24
Determination of the point of zero charge (PZC) of the untreated coffee
residues [116].
was increased to higher than the PZC, the surface of the adsorbent was
negatively-charged and consequently, the electrostatic interaction between
the adsorbent surface and the dye molecule was decreased, resulting in
lower adsorption capacities. The fact that at high pH values, dye removal
can still be observed, although in lower percentages, confirms the exis-
tence of other interactions, such as van der Waals forces and hydrogen
bonding. In extremely high pH values, the dissociation of chlorine atoms
in the monochlorotriazinyl anchor of the dye molecule results in the cre-
ation of a positive charge on the dye molecule which can interact, to some
extent, with the negatively-charged adsorbent. As shown in Figure 5.25,
the trend of the BB adsorption capacity change with pH level was opposite
to that of the RB dye, i.e., the BB adsorption capacity was enhanced by
increasing the pH value. This is due to the positive charge of the dye mol-
ecule after dissociation in the solution which can interact with negatively-
charged surface of the adsorbent at pH values higher than the PZC. The
maximum adsorption capacities of the adsorbent for RB and BB at 25 C
in pH values of 2 and 10, respectively, were reported to be 179 and 295
mg.g
-1
, respectively. Higher adsorption capacities were observed at higher
Search WWH ::

Custom Search