Environmental Engineering Reference
In-Depth Information
0.08
0.07
0.06
0.05
0.04
0.03
ms = 5 g/dm3
ms = 15 g/dm3
ms = 25 g/dm3
ms = 35 g/dm3
ms = 45 g/dm3
Langmuir Isotherm
3.5
0.02
0.01
0
0
0.5
1
1.5
2
2.5
3
4
C
e
(mmol/dm
3
)
Figure 5.16
Langmuir isotherm for the sorption of anionic dye using palm kernel fiber at
various doses [97].
the adsorbent active sites and adsorbate and thus enhanced desorption
rates at higher temperatures. A multi-stage process has been designed in a
similar study in order to minimize the adsorbent mass and contact time,
the two parameters which are critical in the design and cost analysis of a
larger scale [98]. In order to enhance the adsorption properties of the oil
palm fiber, Tan
et al.
[99] carbonized the precursor at 700 C under a pure
nitrogen flow. The resultant char was impregnated with KOH and acti-
vated at 850 C under CO
2
atmosphere. This process resulted in the devel-
opment of a honeycomb-shaped porous structure, probably due to the
intercalation of metallic potassium into the carbonaceous network and
expansion of the carbon material (see Figure 5.17). The surface area of the
resultant activated carbon was determined to be 1354
m
2
.g
-1
with an aver-
age pore diameter of 2.3 nm. The study of the adsorption of methylene
blue by the prepared activated carbon indicated that the maximum uptake
capacity was 276
mg.g
-1
at a pH of 6.5 and an adsorbent loading of 1
g.L
-1
.
As shown in Figure 5.18, the effect of solution temperature was not pro-
nounced for low initial dye concentrations, whereas at higher concentra-
tions, a considerable increase in the adsorption capacity was observed by
raising the temperature. When the temperature of the solution was
increased from 30 to 50 C, the uptake capacity of the adsorbent was
changed from 276 to 384
g.g
-1
, which is quite substantial. This enhance-
ment was attributed to either the increased intraparticle diffusion rate,
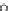

Search WWH ::

Custom Search