Environmental Engineering Reference
In-Depth Information
Table 5.4
Physical and chemical properties of activated carbons from activated
bagasse [5
9].
Carbon
ID
Gasification
Time
Burn-
of
BET SA
(
m
2
g
-1
)
Pore vol.
(
cc.g
-1
)
Av. Pore
Width
(Å)
Ash
(wt%)
Carbon
pH
CC-0
0
0.00
403
0.013
3.66
10.4
2.6
CC-1
1
0.17
614
0.31
0.49
25
6.4
CC-3
3
0.20
737
0.39
0.54
28.7
6.6
CC-5
5
0.27
860
0.46
0.60
34.8
6.8
CC-7
7
0.38
1146
0.67
0.83
44.3
7.1
CC-10
10
0.51
1165
0.70
1.05
46.7
7.4
CC-15
15
0.57
1433
0.91
1.16
61.1
7.4
450
400
350
300
250
200
150
100
50
0
0
50
100
150
200
C
e
(mg dm
-3
)
250
300
350
400
450
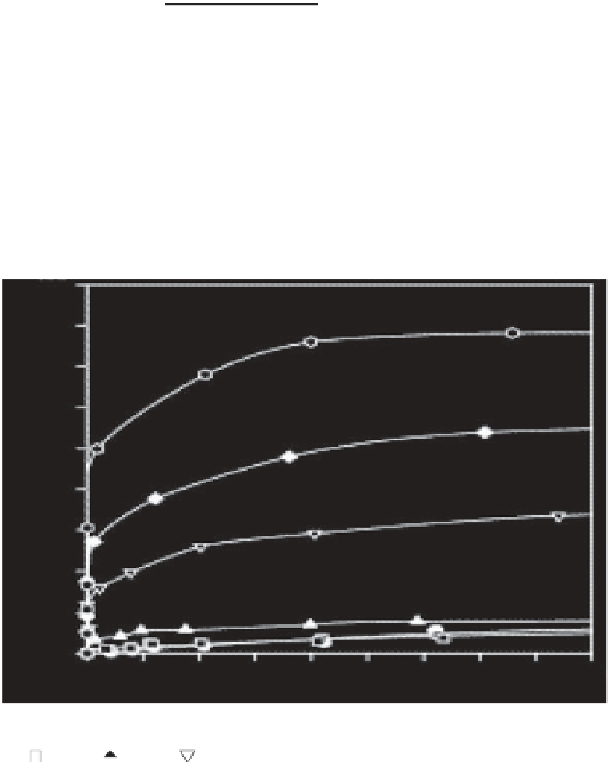
Figure 5.7
Adsorption isotherm of acid blue dye in activated carbon from bagasse
t$$ : CC-3;
: CC-5;
: CC-7;
: CC-10;
: CC-15) [59].
area on the adsorption capacity of the activated carbon. It was also inferred
that an increase in the surface carbon pH will immensely enhance the
adsorption capacity of acid blue dye. It was related to the dissociation
of the dye molecule with a negative charge and its enhanced adsorption
capability on the more basic carbon surfaces. Kadam
et al.
[60] have car-
ried out several modification processes using CaCl
2
, NaOH, NH
4
OH
and steam and concluded that the sample treated with CaCl
2
showed the















































































































Search WWH ::

Custom Search