Geology Reference
In-Depth Information
minerals contain these elements. A combination of silicon
and oxygen is known as
silica
, and minerals that contain
silica are
silicates
. Quartz (SiO
2
) is pure silica because it
is composed entirely of silicon and oxygen. But most sili-
cates have one or more additional elements, as in orthoclase
(KAlSi
3
O
8
) and olivine [(Mg,Fe)
2
SiO
4
]. Silicate minerals
include about one-third of all known minerals, but their
abundance is even more impressive when one considers that
they make up perhaps 95% of Earth's crust.
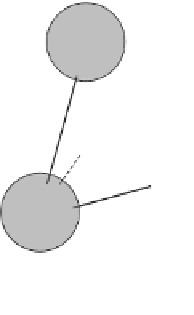
The basic building block of all silicate minerals is the
silica tetrahedron
, consisting of one silicon atom and four
oxygen atoms (
O
O
O
O
S
O
C
Si
O
O
OO
O
O
H
Carbonate
CO
3
Sulfate
SO
4
Silica
SiO
4
(-4)
Hydroxyl
OH
(-2)
(-2)
(-1)
◗
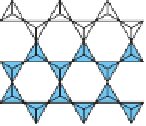
Figure 3.11
Radicals Many minerals contain radicals, which are
complex groups of atoms tightly bonded together. The silica and
carbonate radicals are particularly common in many minerals, such
as quartz (SiO
2
) and calcite (CaCO
3
).
◗
Figure 3.12a). These atoms are arranged so
◗
Figure 3.12
The Silica Tetrahedron and Silicate Materials
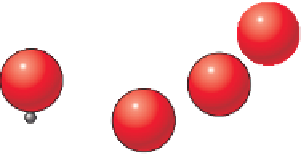
O
-2
View of the silica tetrahedron
from above. Only the oxygen atoms
are visible.
b
O
-2
Si
+4
O
-2
O
-2
(SiO
4
)
-4
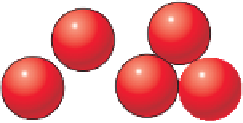
Expanded view of the silica tetrahedron (left) and
how it actually exists with its oxygen atoms touching.
a
Formula of negatively
charged ion group
Example
Isolated
tetrahedra
No oxygen
atoms shared
(SiO
4
)
-4
c
Olivine
Each tetrahedra shares
two oxygen atoms with
adjacent tetrahedra
Pyroxene group
(augite)
(SiO
3
)
-2
Single chain
Continuous
chains
of
tetrahedra
d
Single chains linked by
sharing oxygen atoms
Amphibole group
(hornblende)
(Si
4
O
11
)
-6
Double chain
Continuous
sheets
e
Three oxygen atoms
shared with adjacent
tetrahedra
Micas
(muscovite)
(Si
4
O
10
)
-4
(SiO
2
)
0
Three-
dimensional
networks
All four oxygen atoms
in tetrahedra shared
Quartz
Potassium feldspars
Plagioclase feldspars
f
SiO
2
(
c
-
f
)
Structures of the common silicate minerals shown by various arrangements of the silica tetrahedra.















































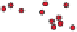









Search WWH ::

Custom Search