Geology Reference
In-Depth Information
could form from 92 naturally occurring elements; however,
several factors limit the number possible. For one thing, many
combinations of elements simply do not occur; no com-
pounds are composed of only potassium and sodium or of
silicon and iron, for example. Another important factor is that
the bulk of Earth's crust is made up of only eight chemical
elements, and even among these eight, silicon and oxygen are
by far the most common. In fact, most common minerals in
Earth's crust consist of silicon, oxygen, and one or more of the
elements in
that have either a positive or negative electrical charge result-
ing from the loss or gain of electrons in their outermost shell.
In addition to ions, some minerals contain tightly bonded,
complex groups of different atoms known as
radicals
that act
as single units. A good example is the carbonate radical, con-
sisting of a carbon atom bonded to three oxygen atoms and
thus having the formula CO
3
and a -2 electrical charge. Other
common radicals and their charges are sulfate (SO
4
, -2),
hydroxyl (OH, -1), and silicate (SiO
4
, -4) (
◗
Figure 3.11).
◗
Figure 3.10.
Geologists recognize mineral classes or groups, each with
members that share the same negatively charged ion or ion
group (Table 3.1). We have mentioned that ions are atoms
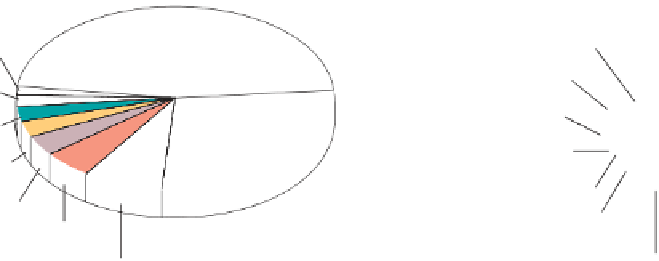
Because silicon and oxygen are the two most abundant
elements in Earth's crust, it is not surprising that many
Earth's crust (by weight)
Earth's crust (by atoms)
All others 0.1%
Oxygen 62.6%
Oxygen 46.6%
All others 1.5%
Magnesium 1.8%
Magnesium 2.1%
Potassium 1.4%
Potassium 2.6%
Silicon 27.7%
Sodium 2.6%
Silicon 21.2%
Sodium 2.8%
Calcium 1.9%
Calcium 3.6%
Iron 1.9%
Iron 5.0%
Aluminum 6.5%
Aluminum 8.1%
Percentage of crust by weight.
Percentage of crust by atoms.
a
b
◗
Figure 3.10
Common Elements in Earth's Crust
a
Source: From Miller, G. T., 1996.
Living in the
Environment: Principles, Concepts, and Solutions
. Wadsworth Publishing. Figure 8.3.
TABLE 3.1
Mineral Groups Recognized by Geologists
Negatively
Charged Ion
Mineral Group
or Radical
Examples
Composition
Carbonate
(CO
3
)
-2
Calcite
CaCO
3
Dolomite
CaMg(CO
3
)
2
Halide
Cl
-1
, F
-1
Halite
NaCl
Fluorite
CaF
2
Hydroxide
(OH)
-1
Brucite
Mg(OH)
2
Native element
—
Gold
Au
Silver
Ag*
Diamond
C
Phosphate
(PO
4
)
-3
Apatite
Ca
5
(PO
4
)
3
(F,Cl)
Oxide
O
-2
Hematite
Fe
2
O
3
Magnetite
Fe
3
O
4
(SiO
4
)
-4
Silicate
Quartz
SiO
2
Potassium feldspar
KAlSi
3
O
8
Olivine
(Mg,Fe)
2
SiO
4
Sulfate
(SO
4
)
-2
Anhydrite
CaSO
4
CaSO
4
.
2H
2
O
Gypsum
Sulfi de
S
-2
Galena
PbS
Pyrite
FeS
2
Argentite
Ag
2
S*
*Note that silver is found as both a native element and a sulfi de mineral.





















Search WWH ::

Custom Search