Geology Reference
In-Depth Information
◗
Figure 17.29
Stalagmites and Climate Change
Stalactites
1
Newly forming layers of
calcite in a stalagmite
contain U
234
(substituting
for calcium).
U
234
decays to Th
230
at a predictable,
measurable rate.
3
U
234
Th
230
Stalagmite
2
5
Time
2
The inside of a stalagmite
is layered like an onion,
showing its incremental
growth.
Time of layer formation
4
The age of each layer can be determined by
measuring its ratio of U
234
/Th
230
. Layer 5 (to
the left) is older than layer 2. It has a lower
U
234
/Th
230
ratio.
a
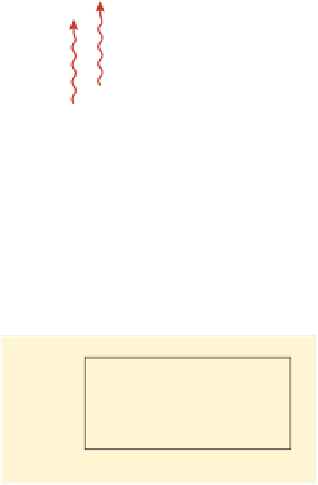
Stalagmites are icicle-shaped structures rising from the fl oor of a cave and are formed by the precipitation of calcium carbonate from
evaporating water. A stalagmite is thus layered, with the oldest layer in the center and the youngest layers on the outside. Uranium 234
frequently substitutes for the calcium ion in the calcium carbonate of the stalagmite. Uranium 234 decays to thorium 230 at a predictable
and measurable rate. Therefore, the age of each layer of the stalagmite can be dated by measuring its ratio of uranium 234 to thorium 230.
3
Water infiltrating the ground
carries pollen and a specific
climate-related O
18
/O
16
- ratio
with it.
Pollinating
plants
1
There is a light isotope
of oxygen, O
16
, and
a heavy one, O
18
.
O
16
- rich
vapor
2
When water (H
2
O) evaporates, it is easier for
O
16
to vaporize than O
18
. Because it is
lighter, the unevaporated water becomes
O
18
- enriched. The warmer the climate, the
more the evaporation and oxygen isotope
separation.
4
The water drips into a
cave and is trapped in
pores between calcite
crystals in the layers of
stalagmites. The pollen
and O
18
/O
16
in the water
records climate conditions
just before the moment of
entrapment.
O
18
O
16
in water
Temperature
b
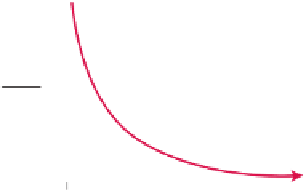
There are two isotopes of oxygen, a light one, oxygen 16, and a heavy one, oxygen 18. Because the oxygen 16 isotope is lighter than the
oxygen 18 isotope, it vaporizes more readily than the oxygen 18 isotope when water evaporates. Therefore, as the climate becomes warmer,
evaporation increases, and the O
18
/O
16
ratio becomes higher in the remaining water. Water in the form of rain or snow percolates into the
ground and becomes trapped in the pores between the calcite forming the stalagmites.















Search WWH ::

Custom Search