Geology Reference
In-Depth Information
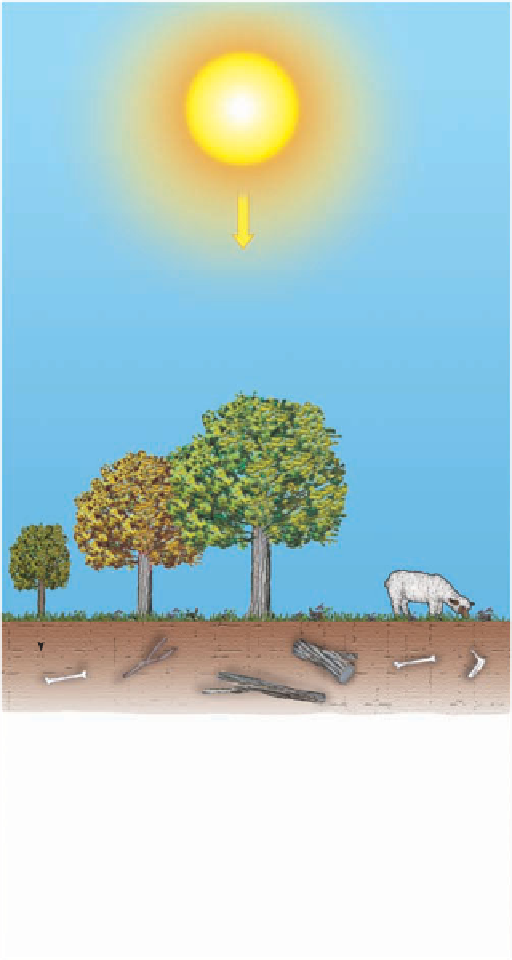
Carbon 14 is constantly formed in the upper atmosphere
when cosmic rays, which are high-energy particles (mostly
protons), strike the atoms of upper-atmospheric gases, split-
ting their nuclei into protons and neutrons. When a neutron
strikes the nucleus of a nitrogen atom (atomic number 7,
atomic mass number 14), it may be absorbed into the nu-
cleus and a proton emitted. Thus, the atomic number of the
atom decreases by 1, whereas the atomic mass number stays
the same. Because the atomic number has changed, a new el-
ement, carbon 14 (atomic number 6, atomic mass number
14), is formed. The newly formed carbon 14 is rapidly assim-
ilated into the carbon cycle and, along with carbon 12 and 13,
is absorbed in a nearly constant ratio by all living organisms
(
14 is not replenished, and the ratio of carbon 14 to carbon
12 decreases as carbon 14 decays back to nitrogen by a single
beta decay step (Figure 17.24).
Currently, the ratio of carbon 14 to carbon 12 is remark-
ably constant both in the atmosphere and in living organ-
isms. There is good evidence, however, that the production
of carbon 14, and thus the ratio of carbon 14 to carbon 12,
has varied somewhat during the past several thousand years.
This was determined by comparing ages established by
carbon-14 dating of wood samples with ages established by
counting annual tree rings in the same samples. As a result,
carbon-14 ages have been corrected to refl ect such variations
in the past.
Tree-ring dating
is another useful method for dating
geologically recent events. The age of a tree can be determined
by counting the growth rings in the lower part of the trunk.
Each ring represents one year's growth, and the pattern of
wide and narrow rings can be compared among trees to
establish the exact year in which the rings were formed. The
procedure of matching ring patterns from numerous trees
and wood fragments in a given area is called
cross-dating
.
By correlating distinctive tree-ring sequences from
living and nearby dead trees, scientists can construct a
time scale that extends back approximately 14,000 years
(
◗
Figure 17.24). When an organism dies, however, carbon
Cosmic
radiation
Figure 17.25). By matching ring patterns to the composite
ring scale, wood samples whose ages are not known can be
accurately dated.
The applicability of tree-ring dating is somewhat limited
because it can be used only where continuous tree records are
found. It is therefore most useful in arid regions, particularly the
southwestern United States, where trees live a very long time.
◗
Neutron capture
Nitrogen 14
Carbon 14
C
14
is absorbed
along with C
12
and
C
13
into the tissue
of living organisms
in a fairly constant
ratio.
TIME SCALE
The geologic time scale is a hierarchical scale in which the
4.6-billion-year history of Earth is divided into time units
of varying duration (Figure 17.1). It did not result from
the work of any one individual, but rather evolved, primar-
ily during the 19th century, through the efforts of many
people.
By applying relative-dating methods to rock outcrops,
geologists in England and western Europe defi ned the major
geologic time units without the benefi t of radiometric dat-
ing techniques. Using the principles of superposition and
fossil succession, they correlated various rock exposures and
pieced together a composite geologic section. This composite
section is, in effect, a relative time scale because the rocks are
arranged in their correct sequential order.
By the beginning of the 20th century, geologists had de-
veloped a relative geologic time scale, but did not yet have
any absolute dates for the various time-unit boundaries.
Following the discovery of radioactivity near the end of the
19th century, radiometric dates were added to the relative
geologic time scale (Figure 17.1).
Because sedimentary rocks, with rare exceptions,
cannot be radiometrically dated, geologists have had to
Soil
When an organism dies, C
14
converts
back to N
14
by beta decay.
Beta decay
Carbon 14
Nitrogen 14
Beta
particle
Proton
Neutron
◗
Figure 17.24
Carbon-14 Dating Method The carbon cycle
showing the formation of carbon 14 in the upper atmosphere, its
dispersal and incorporation into the tissues of all living organisms,
and its decay back to nitrogen 14 by beta decay.



























Search WWH ::

Custom Search