Geology Reference
In-Depth Information
of minerals in pore spaces
is also necessary. The two
most common chemical ce-
ments are calcium carbonate
(CaCO
3
) and silicon dioxide
(SiO
2
), but iron oxide and
hydroxide cement, such as he-
matite (Fe
2
O
3
) and limonite
[FeO(OH)
.
n
H
2
O], are found
in some sedimentary rocks.
Recall that calcium carbonate
readily dissolves in water that
contains a small amount of
carbonic acid, and chemical
weathering of feldspars and
other minerals yields silica
in solution. Cementation
takes place when minerals
precipitate in the pore spaces
of sediment from circulating
water, thereby binding the
loose particles together. Iron
oxide and hydroxide cements
account for the red, yellow,
and brown sedimentary rocks
found in many areas (see the
chapter opening photograph).
We have explained lith-
ifi cation of detrital sediments,
but we have not yet consid-
ered this process in chemical
sediments. By far the most
common chemical sediments
are calcium carbonate mud
and sand- and gravel-sized ac-
cumulations of calcium car-
bonate grains, such as shells
and shell fragments. Com-
paction and cementation also
take place in these sediments,
converting them into various
types of limestone, but com-
paction is generally less ef-
fective because cementation
takes place soon after deposi-
tion. In any case, the cement is
calcium carbonate derived by
partial solution of some of the
particles in the deposit.
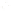
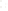
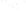
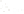
SOURCE OF SEDIMENT
MECHANICAL
WEATHERING
CHEMICAL
WEATHERING
(gravel, sand, silt,
clay-sized particles)
(clay minerals and ions,
compounds in solution)
TRANSPORT
Transport
Transport
Precipitation
from solution
Used by
organisms
Deposition
(detrital sediments)
Deposition
(chemical sediment)
Lithification
Lithification
Detrital sedimentary rocks
(e.g.,sandstone)
Chemical sedimentary rock
(e.g., limestone)
a
b
◗
Figure 6.15
The Origin of Sedimentary Rocks Notice that several steps are involved
in the origin of sedimentary rocks, including weathering, transport, deposition, and
lithifi cation. This illustration simply shows part of the rock cycle in more detail (see Figure
1.14).
























































































































































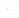


























































































































































































Search WWH ::

Custom Search