Biomedical Engineering Reference
In-Depth Information
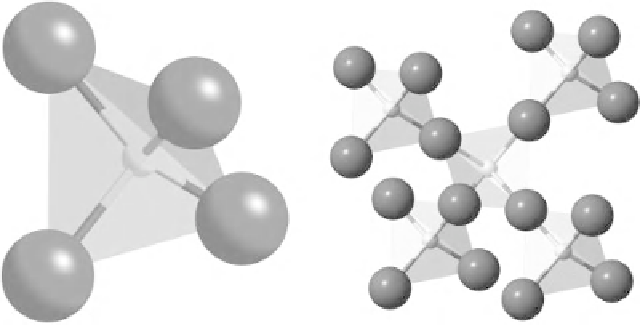
(a)
(b)
Figure 5.1
(a) A single SiO
4
tetrahedron and (b) four tetrahedra linked to a central
tetrahedron through bridging oxygen ions.
the SiO
4
coordination tetrahedron. The tetrahedron is a geometric unit
in which a silicon atom, sitting at its centre, is bonded to four oxygen
atoms, which form the four vertices of the tetrahedron (Figure 5.1a); the
tetrahedral arrangement comes about because of the strong, covalent
character of the Si-O bond.
The starting point for the description of glasses usually begins, follow-
ing Zachariasen [1], with vitreous silica, pure SiO
2
, whose structure is
considered to consist of a three-dimensional network of SiO
4
tetrahedra,
each of which is linked to four other tetrahedra through an oxygen atom
that is common to both tetrahedra. Each tetrahedron is associated with
a node in the network. This is illustrated in Figure 5.1(b).
The network structure of silica is a random network, which is to say
that there is no long-range order or translational periodicity, as indicated
above. Such networks are usually characterized in terms of their ring
size distribution. In a network, it is possible to define rings, whose size
is determined by the number of nodes one traverses before arriving back
at the node from which one started. Random networks will, in general,
contain rings of all possible sizes, in contrast to crystalline structures,
which, in general, contain rings of only one or two discrete sizes. For
example, quartz, a crystalline polymorph of SiO
2
, contains rings of six
and eight tetrahedra, whereas cristobalite, a different silica polymorph,
contains only six-membered rings (Figure 5.2).