Biomedical Engineering Reference
In-Depth Information
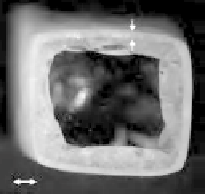
Crystalline surface layer
T
p,a2
T
p,a1
T
p,b
10 mm
frit
powder
T
g
400
450
500
550
600
650
700
Temperature (
°
C)
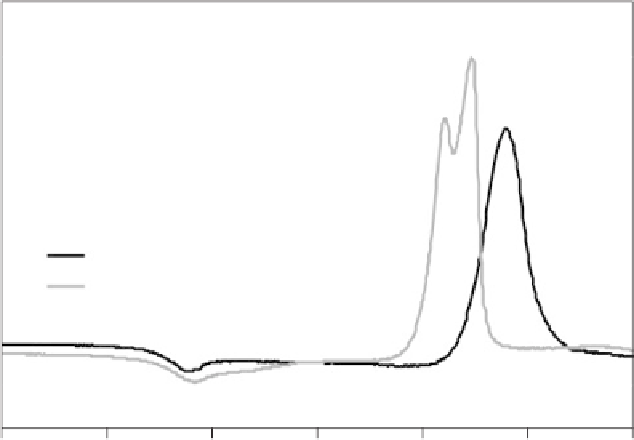
Figure 4.12
DSC traces of frit (particle size about 1 mm) and fine powder of a phos-
phate invert glass in the system P
2
O
5
-CaO-MgO-Na
2
O-TiO
2
with 34.8 mol%
P
2
O
5
. Traces show glass transition (
T
g
) and crystallisation temperatures (
T
p,a1
,
T
p,a2
and
T
p,b
). Inset shows surface crystallisation on glass monolith after heat
treatment for 30 minutes at 540
◦
C.
When comparing the DSC traces of frit (i.e. particles obtained by
quenching the glass melt in water) and milled fine powder of a phosphate
invert glass (34.8 mol% P
2
O
5
) in Figure 4.12, two main differences are
obvious: the crystallisation peak of the fine powder appears at a lower
temperature than that of frit; and it actually consists of two overlapping
peaks, compared to only one visible peak for glass frit. The reason for
the double peak is that the glass crystallises into two different phases
(identified as calcium pyrophosphate, Ca
2
P
2
O
7
, and a mixed calcium
magnesium pyrophosphate, CaMgP
2
O
7
), which are resolved as separate
peaks in the DSC trace of fine glass powder but not for glass frit. The
decrease in crystallisation temperature with decreasing particle size (from
coarse frit to fine powder), on the other hand, often suggests a surface
crystallisation mechanism. Surface crystallisation means that, upon heat
treatment, the crystals start growing from the outer surface of the glass
towards the centre, rather than throughout the bulk of the material
as in volume crystallisation. Indeed, if a glass monolith of the same