Biomedical Engineering Reference
In-Depth Information
O
−
O
O
O
P
O
P
O
−
O
Ca
2
+
O
−
O
O
P
O
P
O
O
−
O
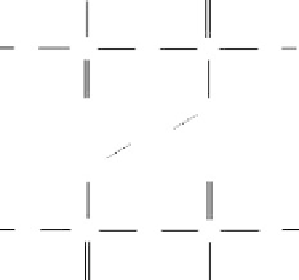
Figure 4.4
Simplified chelate structure of ionic cross-links between phosphate
chains.
Corresponding to their structure, phosphate glasses can be divided
into different groups [3]. Ultraphosphate glasses contain more than
50 mol% P
2
O
5
, that is, less than 50 mol% of network modifier oxides,
and accordingly they consist of two- to three-dimensional phosphate
networks. Polyphosphate glasses contain less than 50 mol% P
2
O
5
,and
they are built of phosphate chains and rings, with the chain length
decreasing with decreasing phosphate content. In between these are
metaphosphate glasses that contain 50 mol% P
2
O
5
. Their structure also
consists of chains of infinite length or rings, and is therefore better
described as consisting of entangled 'molecular' or 'polymeric' chains,
rather than being an actual 'network'. The linear phosphate chains (but
also the rings) in the structure of metaphosphate and polyphosphate
glasses can be ionically connected to one another through ionic bonds
via modifier cations; divalent or higher valent cations (e.g. Ca
2
+
,Fe
3
+
or
Ti
4
+
) can serve as ionic cross-links between the NBOs of two individual
chains, and it has been suggested that such a cross-link could take the
form of a metal chelate structure (Figure 4.4). Owing to difficulties in
obtaining exactly the 50 mol% P
2
O
5
stoichiometry, most metaphosphate
glasses are actually long-chained polyphosphate glasses [4].
Phosphate invert glasses (pyrophosphate glasses, less than 33.3 mol%
P
2
O
5
) are formed by orthophosphate (PO
4
3
−
) and pyrophosphate
groups (P
2
O
7
4
−
; phosphate dimers) exclusively (Figure 4.5). For glasses
with lower phosphate contents, isolated orthophosphate groups are
present. In these cases, the glassy state is caused neither by a rela-
tively stiff network nor by entangled chains but by the interaction of