Biomedical Engineering Reference
In-Depth Information
4.2 MAKING PHOSPHATE GLASSES
Phosphate glasses are commonly produced by mixing the glass precursors
(powders of phosphates, oxides and carbonates; sometimes phosphoric
acid is used as a phosphate source) and melting at high temperatures
in a platinum crucible. Alumina crucibles should be avoided in order
to prevent aluminium contamination of the glasses, as aluminium is
a neurotoxin and negatively affects bone mineralisation. The melting
temperatures of phosphate glasses tend to be lower than those of
bioactive silicate glasses, and are usually between 800 and 1300
◦
C,
depending on the glass composition. After the material has melted
completely, the melt is cooled by either quenching quickly between
metal plates or by pouring into water. In order to obtain bulk glass
(monoliths), the hot melt is poured into preheated graphite or metal
moulds, placed into a furnace preheated to the transition temperature
of the glass and annealed, that is, allowed to cool down to room
temperature slowly in order to reduce stresses in the glass.
An alternative route for making glasses is by a sol-gel route.
However, sol-gel processing of phosphate glasses is relatively new [2],
the glasses produced seem to be more fragile than bioactive silicate
sol-gel glasses (Chapter 3) and they are very soluble, possibly too
soluble at the time of writing for most biomedical applications.
4.3 PHOSPHATE GLASS STRUCTURE
Like silicate glasses, phosphate glass structure does not show any long-
range order or significant symmetry of atomic arrangement, but they
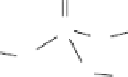
do have short-range order. The glass-forming component in phosphate
glasses is P
2
O
5
, and the basic unit in the phosphate glass structure is the
orthophosphate (PO
4
3
−
) tetrahedron (Figure 4.1), which is a phosphorus
atom surrounded by four oxygen atoms. As one of the oxygen atoms is
connected to the phosphorus atom by a double bond, only the three other
oxygen atoms can act as 'bridges' to other orthophosphate tetrahedra.
By forming such bridges, individual orthophosphate tetrahedra can be
O
P
O
O
O
Figure 4.1
Basic phosphate tetrahedron in glass structures.