Biomedical Engineering Reference
In-Depth Information
Oxygen
Microsphere
Collection
Barrel
Acetylene/oxygen flame
Flame Sprayer
REAS Glass
Microspheres
REAS Glass
Particles
Acetylene
REAS Glass Powder in N
2
Gas
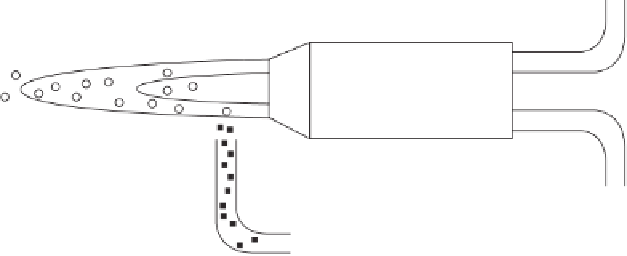
Figure 13.3
Schematic of the system used to produce glass microspheres by
spheroidizing a glass powder in a gas burner (flame spheroidization). (Reprinted
with permission from [4]. Copyright (1994) Trans Tech Publications.)
REAS glasses form over a fairly broad compositional range in each
system, which is a desirable feature. Note also that these glasses can
contain large amounts of the various RE oxides, from a low of 32wt%
to a high of 69wt%, depending upon the specific RE oxide. Yttrium,
while not strictly a RE, is included in Figure 13.2 since its properties are
close to those for the RE elements.
To make microspheres, the glass is crushed to a powder of the
generally desired size and the powder is feed to a gas burner as shown
in Figure 13.3. The glass particles melt in the flame and the molten
droplets become spherical (Figure 13.1b and see Figure 15 in colour
section) owing to surface tension. After cooling, the microspheres are
screened to the exact size needed for the specific application. Many of
the REAS glasses can also be pulled into fibers ranging in diameter from
10 to 5000
μ
m.
13.3.2 Properties
As would be expected, the properties of REAS glasses depend some-
what upon the particular RE element present in the glass. Some selected
properties for REAS glasses containing yttrium (Y), samarium (Sm),
holmium (Ho), or dysprosium (Dy) are given in Table 13.1. The
line labeled ''RE
2
O
3
content'' in Table 13.1 denotes the approximate
minimum-maximum amount of RE oxide for each system. The density
of the REAS glasses in Table 13.1 increases with increasing RE
2
O
3