Biomedical Engineering Reference
In-Depth Information
(a)
(b)
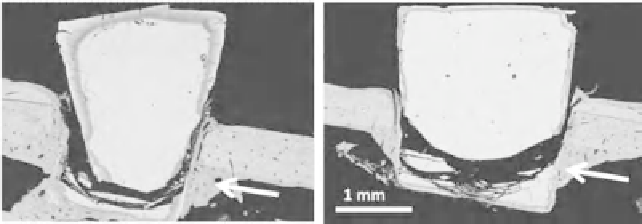
Figure 11.2
Cross-sections of cones of (a) 45S5 glass and (b) S53P4 glass after
eight weeks in rat femur. The arrows point out the new bone. (Expanded view of
Figure 11.2 in [3]. Copyright (2010) Society of Glass Technology.)
good contact with the bone, as indicated by the implant failure within
the glass.
Glass composition has a great effect on bioactivity and processability.
Chapter 2 described the effect of silica content on bioactivity and how
network modifiers could be replaced with others to change properties;
for example, replacing calcium oxide with strontium oxide has been
found to enhance bone formation.
The number of different network modifiers can also be changed. To
make porous glasses (Chapter 12), coatings (Chapter 8) or fibres from
melt-derived glasses, the glasses have to be worked above their glass
transition temperature. The low silica content and high lime content of
the first bioactive glasses make them prone to crystallise when they are
above the glass transition temperature. This is the reason why current
commercial bioactive glasses are mainly particulates. Glasses containing
more oxides can increase the temperature difference between the glass
transition temperature and the crystallisation onset temperature, creating
a wider operating window. K
2
O, MgO, B
2
O
3
and Al
2
O
3
can be added
to the glasses [2, 4, 5] to do this. The compositions of the studied glasses
were chosen with the help of statistics, thus enabling the modelling of
the measured properties as functions of the oxide composition of the
glass. Today, models of
in vitro
and
in vivo
reactivities as well as models
of the hot working properties are available [2, 4-6]. These property
models can be utilised to tailor compositions for controlled reactivity in
any product form desired.