Biomedical Engineering Reference
In-Depth Information
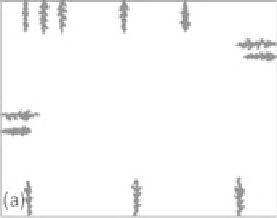
Figure 7.2
Controlled crystallization on the surface of the base glass in the
development of glass-ceramics: (a) nucleation and (b) growth. (Reprinted with
permission from [2]. Copyright (2006) W. Holand.)
desired size, the base glass is cooled down to stop them from growing
further. The condition of the glass that has been achieved and the size
of the crystals is preserved by freezing the material. This is how the
microstructure of glass-ceramics is produced.
In the next section, we will look at different glass-ceramics with
very special characteristics. We will explain their chemistry, describe
the components that are used to start the crystallization process, and
examine the crystallized parts of the material, which are responsible for
producing the special characteristics of the glass-ceramic.
7.3 A GLASS-CERAMIC THAT HARDLY EXPANDS
WHEN HEATED
The combination of SiO
2
-Al
2
O
3
-Li
2
O is suitable for making glass,
which can be used to produce glass-ceramics that hardly expand when
they are heated. This is important when making precision shapes, as the
amount of expansion of other systems is difficult to control to exact
measurements. These three oxides form what is called a glass formation
system and can be supplemented with a wide range of other chemi-
cal compounds. The substances that cause the crystallization process
(nucleating agents) are the most important compounds. TiO
2
and ZrO
2
are usually used for this purpose. When the base glass is heated, these
compounds react and form a first crystal structure on which the desired
crystal structure then grows when the glass is heated again. Over many
years, researchers in the USA, Europe, and Japan developed crystalliza-
tion processes in which SiO
2
mixed crystals (also called solid solutions)
could be produced. The main aim of their work was to produce an SiO
2
crystal structure that would not expand when the material was heated