Biomedical Engineering Reference
In-Depth Information
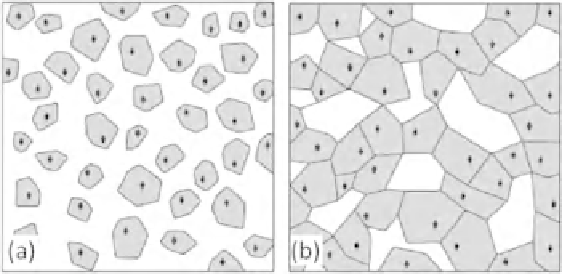
Figure 7.1
Controlled internal crystallization in glasses for producing glass-
ceramics: (a) nucleation of crystallites in a glass; and (b) crystals grow and join
at grain boundaries. (Reprinted with permission from [2]. Copyright (2006) W.
Holand.)
In internal nucleation and crystallization, centers, or nucleation sites,
from which the crystallization process starts are required. These sites are
formed by chemical compounds that are added to the base glass. The
chemical compounds, which are called nucleating agents, speed up the
beginning of a process. We will explain what these nucleating agents are
all about in the next few paragraphs. These substances form a crystalline
phase, a foreign substrate, on which the actual crystal phase grows. This
phenomenon is shown in Figure 7.1.
Glass-in-glass phase separation provides another way of starting the
internal crystallization process within a base glass. During the separation
of the glass into different phases, specific ions are concentrated into one
phase, which enables nuclei and then crystal to form.
In addition to the method involving internal crystallization, the
process of surface nucleation and crystallization is used to produce
glass-ceramics. A diagram of the mechanism is shown in Figure 7.2.
Glass is crushed to form a powder, and the surface of the glass powder
grains is activated to produce crystals. These crystals then proceed to
grow inwards from the surface of the glass particles.
For both methods to work, the chemical composition must be just
right, and the base glass has to be heated to a very high temperature in
the region of 1000
◦
C. By heating the glass, the processes of nucleation
and crystallization are started and kept under control. The thermal
energy (heat) makes molecules move around in the base glass and
stimulates the growth of crystals in very specific areas. This is clearly
shown in Figures 7.1 and 7.2. When the crystals have reached the