Environmental Engineering Reference
In-Depth Information
10.2
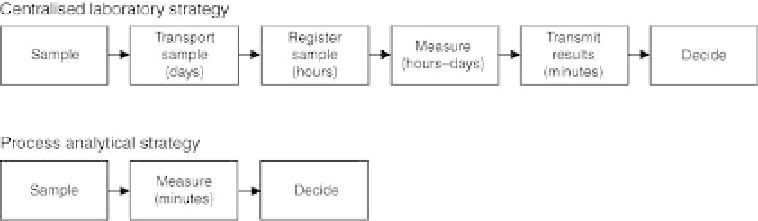
Comparison of analytical strategies for process monitoring.
also widespread in other industries. In 2005, the US Food and Drug
Administration produced industry guidance in which PAT was defined as a
system for designing, analysing and controlling manufacturing through
timely measurements during processing of critical quality and performance
parameters of raw and process intermediates (FDA, 2005). The goal is to
monitor and control the process on-line as early as possible in the process
and in real time at strategically selected process locations with steps that
ensure the quality of the final product. The term 'analytical technology' in
PAT refers to analytical chemical, physical, microbiological, mathematical,
data and risk analysis conducted in an integrated manner. The term 'quality'
of a product has the meaning of the final quality of various industrial
processes; it can either be the concentration, pureness, strength, or similar of
the processed products. According to the FDA guidance 'Quality cannot be
tested into products; it should be built-in or it should be by design'. Product
quality and/or quantity have to be optimised during the ongoing process.
10.3.3 Control strategies: central laboratory versus on-line
control
Traditionally, monitoring and quality control of production processes has
been based on a centralised laboratory approach. Samples are collected
from a process stream and sent to an analysis laboratory where sub-
sampling, sample preparation and chemical analyses are carried out, often
in an optimised manner, allowing multiple samples to be analysed in the
same run. The time span for the primary sampling was taken from the point
of the process stream until the analytical result had been produced and
approved; this could span from several hours to days depending on the
laboratory infrastructure and routine (Mortensen, 2006) (Fig. 10.2).
It is clear from Fig. 10.2 that the analytical strategy has to be changed
from the centralised laboratory approach to the process analytical strategy if
the biogas plant operator wants to benefit from the analytical results and use
them as true process regulation parameters instead of just 'delayed' quality
control parameters. In fact, the quality control task is moving from the
Search WWH ::

Custom Search