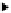Environmental Engineering Reference
In-Depth Information
Some chemical and physical parameters are critical to the hydrocarbon yields
[51]. These include temperature, pressure, residence time, selection of solvent and
catalyst, as well as their concentrations. Intensive research has been carried out to
optimize these parameters to increase hydrocarbon oils yield. Influence of the tem-
perature, residence time, and concentrations of catalysts on the oil yield have been
found to follow a volcano-type pattern in which the hydrocarbon oil yield reaches a
maximum in the intermediate range and drops after any further increase, while the
pressure shows a positive correlation with the hydrocarbon oil yield [53]. On the
other hand, the addition of solvent, catalyst, and reducing gas have been demon-
strated to improve the yield of hydrocarbon oil and prevent the formation of solid
char condensed by the monomers formed in the depolymerization [51]. Catalysts
such as NaOH and H
2
SO
4
are employed to decrease the required reaction tempera-
ture and enhance reaction kinetics. A solvent is usually added to prevent the mono-
mer units from re-polymerizing or condensing into undesirable solid chars. Alcohols
with a short carbon chain such as methanol and ethanol are recognized as favorable
solvents due to the lowest formation of solid char. Furthermore, reducing gases such
as H
2
and CO are used to increase the ratio of hydrogen to carbon in order to facili-
tate an efficient degradation of the oxygen-containing functional groups.
Direct liquefaction of biomass has been tested at a pilot scale in several coun-
tries such as Denmark, Germany, and the USA [54]. The CatLiq® technology
developed by the Danish company SCF Technologies has been successfully
scaled up in a pilot facility in Copenhagen, which is currently one of the largest
direct liquefaction facilities in the world. It processes 20 L hr
-1
of dried distillers
grains with solubles (DDGS), which are the crude protein, crude fiber, and crude
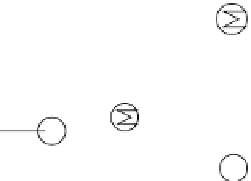
fat generated from first-generation ethanol production. The schematic flow of the
CatLiq process is shown in Figure 3.7. First, the feed from the feed tanks is pres-
surized by the feed pump and preheated by the feed heater. On the other hand, the
circulation pump provides a high flow rate for a uniform distribution of heat and
instantaneously heats up newly added feed. The feed then passes through a trim
heater and enters a fixed-bed reactor filled with a zirconia-based catalyst at sub-
critical condition (280-370°C and 250 bars) for liquefaction. Water is used as
Tr im heater
Reactor
Oil
Feedstock
Water
Soild
Feed heater
Feed pump
Pressure
reduction
Separator
Cooler
Circulation
pump
Figure 3.7
Flow diagram representing the processes in a direct liquefaction plant. Reproduced
from [55], with permission from Elsevier.