Environmental Engineering Reference
In-Depth Information
temperatures lower than that required under conventional thermal means (180°C
in comparison to 250°C). This reduces the need for the addition of aggressive
chemical agents with the additional benefit of preferential depolymerization to
glucose.
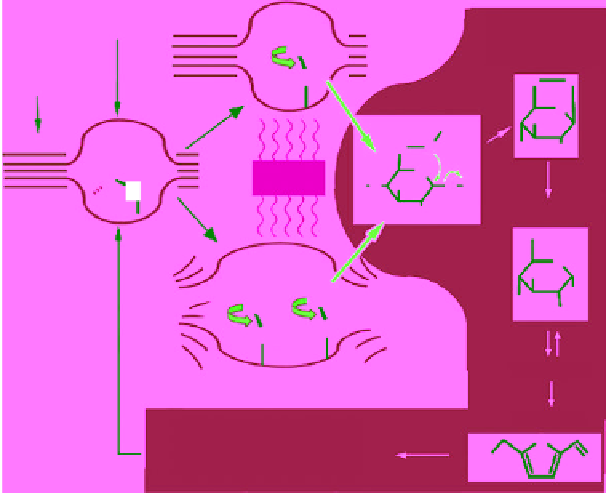
This has been reported by Fan
et al.
[[41], whereby it is believed that the method
of heating through microwave interaction (through dipolar polarization) with
celluloses CH
2
O(6)H mean that, above 180°C, these CH
2
OH groups could be
involved in a localized rotation in the presence of microwaves. The latter act in
similarly to 'molecular radiators', allowing the transfer of microwave energy to
the surrounding environment.
With the limited presence of water inside the rigid cellulose framework, this is
likely to involve collisions between the CH
2
OH groups and the anomeric C1 of
the same glucose ring, thus forming levoglucosan. The latter can easily hydrolyze
to glucose, which is thought to be directly related to microwave activation of the
CH
2
OH pendant groups. This is summarized as the proposed decomposition
mechanism in Figure 3.6.
(a)
(b)
H
2
O
H
2
O
H
2
O
H
2
O
Amorphous
region
OH
Levoglucosan
CH
2
H
2
C
O
OH
O
OH
H
H
2
C
O
OH
OH
O
OH
OH
+H
2
O
MW
RO
OH
OR'
CH
2
Glucose
OH
CH
2
OH
O
OH
OH
OH
OH
OH
OH
CH
2
CH
2
OH
OH
H
2
O
Fructose
H
2
O
H
2
OH
2
O
HMF
O
Acids & aldehydes
HO
O
Figure 3.6
Proposed mechanism for the microwave decomposition of cellulose. Representation
of the cellulose-microwave interaction as a function of temperature: (a) mechanism of CH
2
OH
group activation; (b) scheme of cellulose degradation toward acids and aldehydes. Reproduced
with permission from [41]. Copyright © 2013, American Chemical Society.