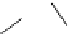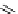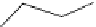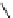Environmental Engineering Reference
In-Depth Information
OH
HO
NH
2
O
O
O
O
NH
HO
OH
H
3
C
O
x
y
Figure 6.19
General formula for chitosan and chitin (in the case of chitin y = 0).
additive to thicken and stabilise foods and pharmaceuticals. Since it can be shaped
into fibres the textile industry also utilises chitin, especially for socks as it is
claimed that chitin fabrics are naturally anti-bacterial and anti-odour (
http://www.
swicofil.com/products/055chitosan.html)
. Chitin can also be used as a binder in
dyes, fabrics and adhesives and, in some processes, to size and strengthen paper.
Chitosan
is produced commercially by deacetylation of chitin. It is a linear
polysaccharide composed of randomly distributed β-(1-4)-linked d-glucosamine
(deacetylated unit) and
N
-acetyl-d-glucosamine (acetylated unit). The degree of
deacetylation in commercial chitosans is in the range 60-100% (Figure 6.19).
The amino group in chitosan has a p
K
a
value of about 6.5. This means that chi-
tosan is positively charged and soluble in acidic to neutral solutions with a charge
density dependent on pH and the deacetylation extent. In other words, chitosan
readily binds to negatively charged surfaces such as mucosal membranes. Chitosan
can also enhance the transport of polar drugs across epithelial surfaces, and is
biocompatible and biodegradable. Purified qualities of chitosan are available for
biomedical applications.
Chitosan possesses flocculating properties, which are used in water processing
engineering as a part of the filtration process. It can remove phosphorus, heavy
minerals and oils from the water. In the same manner, it is used to clarify wine (as
a substitute for egg albumin) and beer.
6.3.6
Proteins
6.3.6.1
Molecular Structure
Proteins are much more complex polymers than carbohydrates; there are 20 com-
mon monomers (in fact 22 proteinogenic, but only 20 are encoded by the universal
genetic code) with polymeric chains composed of amino acids (Figure 6.20, left).
From these monomers all kinds of secondary interactions can arise: Van der Walls,
ionic, hydrogen bonding and hydrophobic interactions and, through a redox
mechanism involving the amino acids bearing sulphur, a reversible covalent disul-
phide bonding is also possible.
As a result of this complicated molecular structure, proteins are organised in an
intricate macromolecular organisation with a primary structure (the amino acid