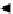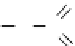Environmental Engineering Reference
In-Depth Information
OCS
-
Na
+
Cell
OH +
CS
2
+
NaOH
Cell
+
H
2
O
Fast
SNa+H
+
Na
+
Cell
OH C
S
Cell
OC
S
SH
+
Slow
Cell
OH
+CS
2
Figure 6.12
Main reactions taking place in the viscose process for cellulose regeneration.
O
H
2
SO
4
Cell OH
+
HNO
3
Cell ON
+ H
2
O
O
Figure 6.13
Synthesis of cellulose nitrate.
sheets or threads or individual beads. On being plunged into a water solution
containing sulphuric acid and other additives, cellulose xanthate is hydrolysed
and converted into cellulose once more (Figure 6.12).
If the extrusion profile is a tiny cylinder, the obtained product is a continuous
thread for textile applications. The industrial name of this product is Rayon®, or
'artificial silk', a brilliant fabric with good dying properties.
The viscose process has some variants. Depending on the quality of the cellu-
lose and the composition of the regenerating bath, special high-added-value prod-
ucts can be obtained including the so-called
modal-polynosic
fibres, or the
modal-high wet modulus
fibres. If the extrusion profile is a sheet, the obtained
product is Cellophane®. Again, some additives are added to the hydrolysing bath
to obtain the transparency and plastic-like aspect of the sheet. Even though the
tensile strength of this product is high, the shearing resistance is low which makes
it an excellent film for packaging and conditioning of biscuits or candies.
The viscose process has been progressively abandoned (but not totally) due to
environmental concerns as CS
2
is toxic and can easily cause an explosion.
However, it remains historically important in the field of the chemistry of cellu-
lose. The viscose process has set standards of variety, quality and cost that any
new process must at least equal, or even surpass. If this is not possible, then it is
only the safety and environmental restrictions which may cause the viscose
process to be totally abandoned (Figure 6.13).
The cellulose carbamate process, which uses urea as modifying agent, repre-
sents a greener alternative to the xanthate process [10]. However, the process
remains to be commercialised.