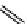Environmental Engineering Reference
In-Depth Information
the acid number decreases. The saponification number is a measure of the total
carboxylic groups. The color of the rosin is often a detrimental factor in many
applications; a color intensity increase correlates to a decrease in quality [141].
The softening point is associated with the glass transition temperature of the rosin,
which directly influences potential applications.
5.5.2
Epoxy Resins from Rosin
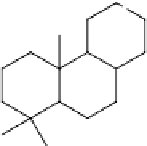
The chemical functionality, in particular of the abietic-type acids, lends rosin to a
number of functionalizations that afford appropriate reactivity for use in epoxy
resins. Gum rosins contain approximately 90% abietic-type acids, and many
chemical modifications can be efficiently designed around this chemistry [145].
Accessing isomers can be facilitated by simple acid-catalyzed isomerization
[141]. Outside the scope of this discussion, dehydrogenation of abietic-type acids
produces dehydroabietic acid with an aromatic ring capable of typical aromatic
substitutions, such as acylation, sulphonation, and nitration [141]. The Diels-Alder
product of abietic-type acids, in particular levopimaric acid, with maleic anhydride
has received much attention as a curing agent for epoxy resins. This Diels-Alder
reaction is shown in Scheme 5.6 [118, 147-151]. The cure kinetics and thermal
mechanical properties of these agents are comparable to their fossil-based coun-
terparts, and they exhibit slightly better
T
g
[147, 150]. However, the degradation
temperatures for these systems are slightly below those of their fossil-based
counterparts [147, 150]. A two-step process to produce epoxy curing agents from
O
OH
Acrylic acid
Acrylopimaric acid
O
Levopimaric acid
OH
HO
O
O
Diels-Alder reaction
O
OH
O
O
O
Maleopimaric acid
O
O
OH
Maleic Anhydride
O
Scheme 5.6
Diels-Alder products of reaction between levopimaric acid with acrylic acid and
maleic anhydride [118].