Environmental Engineering Reference
In-Depth Information
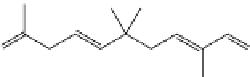
Caryophyllene
*
*
ROMP
poly(Caryophyllene)
Humulene
*
*
poly(Humulene)
Scheme 5.5
Examples of linear thermoplastics achievable by ROMP of the sesquiterpenes
caryophyllene and humulene [123].
More complex terpenes are also available, and caryophyllene and humulene are
two examples of sesquiterpenes that contain three isoprene units and are found in
both clove oil and hop oil [123]. A recent study showed the ability to use ring-
opening metathesis on these sesquiterpenes to produce thermoplastic polymers
with molecular weights of the order 10
4
Da. Scheme 5.5 illustrates the complex
structure of these sesquiterpenes and the resulting thermoplastic backbones [123].
The resulting polymers had unsaturation sites that can be targeted for cross-
linking or can be hydrogenated to ensure thermoplastic behavior [123]. The
polymers form soft materials with low
T
g
(-15 to -50°C), which makes them
attractive for film and coating applications [123].
5.4.5
Terpenoids
Terpenoids are similar to terpenes in that their structure is based on the C5 iso-
prene building block. However, while terpenes are hydrocarbons, terpenoids also
contain functional groups. Terpenoids occur naturally in plant oils or can be pro-
duced via chemical modification of terpenes. Two common terpenoids are carvone
and menthol. Recent work by Lowe
et al.
focused on carvone, a terpenoid found
in spearmint and caraway oils [124]. The work demonstrated a pathway to poly-
esters through ring-opening polymerization of lactone [124]. The process utilized
a Baeyer-Villiger oxidation to modify carvone (a cyclic ketone) into a lactone,
which was then polymerized through a facile ring-opening mechanism [124]. The
resulting polymers had molecular weights in the tens of thousands and had avail-
able double bonds for post-polymerization functionalization [124]. This work
illustrates a pathway to thermoset materials from the terpenoid/terpene platform.