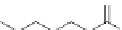Environmental Engineering Reference
In-Depth Information
the cleavage of the fatty acid chain from the glycerol. Several free fatty acids and
their esters are of value as oleochemicals, including ricinoleic acid, oleic acid,
lauric acid and palmitic acid, and the chemicals formed from these acids are
referred to as oleochemicals. The longer-chained saturated acids (lauric and
palmitic) are mainly used for surfactant production or additives to personal care
formulations. The carbon-carbon double bonds in oleic and linoleic acids are
used for further functionalisation of the chain, such as epoxidation, hydration and
alkylation, but can also be used for chain scission, typically via ozonolysis
(forming azelaic acid, used for formation of nylon-6,9) or ethenolysis (Figure 4.18)
[219]. Unsaturation can be used to connect two fatty acid chains together, the
most common being a C
36
dimer acid used in adhesives, polyesters and
polyurethanes [220]. These are often further modified by reduction to the dimer
diol, which is also useful for polymer applications [116]. Ricinoleic acid is another
fatty acid of high value for chemical production, both as a result of its β-hydroxy
alkene moiety and its high abundance in castor oil triglycerides, typically between
85 and 90% [221]. The most important products of ricinoleic acid are the result of
chain scission (alkali fusion or pyrolysis), giving sebacic acid, 10-hydroxydecanic
acid or 10-undecenoic acid (Figure 4.18) depending upon the conditions used,
while also producing one equivalent of 2-octanol, 2-octanone or heptanal,
respectively [222, 223]. The larger scission products are typically used for the
production of various nylons (nylon-4,10, nylon-6,10, nylon-10,10, nylon-11)
[116]. Additionally, ricinoleic acid or its esters can be hydrogenated or dehydrated
to various useful derivatives (Figure 4.18) [224]. The carboxylic acid function can
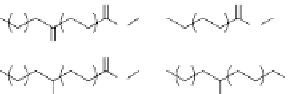
O
O
Sebacic acid
10-hydroxyundenanoic acid
HO
HO
OH
OH
+
OH
+
O
O
O
2-octanol
2-octanone
O
Azelaic acid
O
O
10-undecenoic acid
OH
HO
OH
O
+
+
O
Ricinoleic or
oleic acid
HO
H
Heptanal
Perlargonic acid
O
O
OH
O
O
4
5
8
O
O
HO
O
O
OH
4
5
4
5
Dimer acid
O
O
OH
Hydrogenated ricinolate
Figure 4.18
Triglyceride platform: example oleochemicals derivable from oleic and
ricinoleic acid.