Biology Reference
In-Depth Information
PDE4A
PDE4A
A
PDE4B
PDE4B
B
PDE4D
D
PDE4D
1,2 + Cat
Cat
2 + Cat
1,2 + Cat
Cat
2 + Cat
1,2 + Cat
UCR1
UCR2
Catalytic Domain
2 + Cat
Catalytic Domain
UCR2
Catalytic Domain
Cat
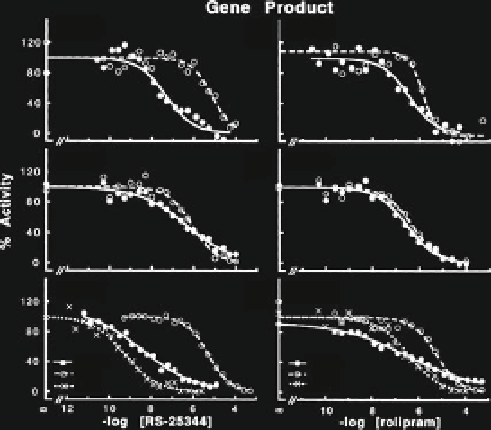
Fig. 3 Comparison of the potency of inhibition of cloned forms of PDE4 gene products with
rolipram and RS-25344. Representative data showing the inhibition of PDE4A, 4B or 4D activity
(
gene product A, B and D
, respectively) by RS-25344 (
left
) or rolipram (
right
). A cartoon model
depicts the functional domains in the respective long (1,2
þ
Cat) forms of PDE4 proteins containing
UCR1, UCR2, and the catalytic domain, the short (2
þ
Cat) forms of the enzymes containing
UCR2 and the catalytic domain, as well as that of the isolated catalytic domain (Cat). Each data
point represents the average of quadruplicates, and the curves are plotted as a percentage of the
difference between the maximum and minimum rates of each enzyme. Reprinted from Saldou
et al. (
1998
)
the full-length enzyme, but stronger potencies are exhibited in constructs where
UCR2 is conjoined with the catalytic domain (Fig.
3
) (Saldou et al.
1998
). Like-
wise, Burgin and coworkers recently reported that two inhibitors (RS25344 and
PMNPQ) are 10,000 times more potent toward inhibition of PDE4D7 than against
the isolated PDE4 catalytic domain (Burgin et al.
2010
). It is now clear that these
truncations remove important regulatory/oligomerization domains in the holoen-
zyme that affect interdomain contacts and impact the conformation and/or func-
tions of the catalytic domain (Burgin et al.
2010
; Houslay
2001
; Houslay and
Adams
2003
,
2010
; Richter and Conti
2002
). It has been observed that some
variants of PDE4s, termed short forms, have lower affinity for rolipram and kinetic
properties distinct from those of longer forms (see below). Since short and long
forms are expressed in a tissue- and cell-specific manner, this must be considered
when evaluating the potency and biological action of selected compounds.
Likewise, the inhibitory potency of the PDE5 inhibitor, vardenafil, for the PDE5
holoenzyme exceeds that of sildenafil by 10- to 40-fold (Blount et al.
2004
,
2006
).