Biology Reference
In-Depth Information
N321
E339
Q369
H164
H160
cAMP
H200
Zn
P
OH
-
D201
Mg
H
2
O
H
2
O
H
2
O
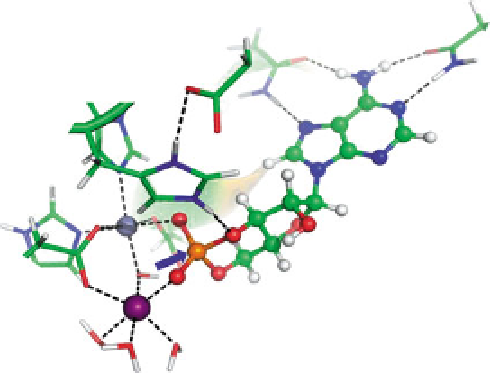
Fig. 2 Scheme proposed for mechanism of hydrolysis of cAMP by PDE4. The adenine (shown in
green
and
blue
at
upper right
) is coordinated through hydrogen bonds (
dashed lines
) to Gln369 and
Asn321. The phosphorous in the cyclic phosphate ring is approximated to the hydroxyl ion that
bridges the two metal ions [Zn (in
steel
) and Mg (in
purple
)], and the
blue arrow
indicates the attack
of this hydroxyl at that phosphorous (shown in
orange
) to break the ring. Residues coordinating the
metal ions are shown. The Glu339/His160 hydrogen-bond relay that fosters interaction of His160
with one of the oxygens (
red
) in the cyclic phosphate ring is shown. This figure was kindly provided
by Prof. Chang-Guo Zhan, College of Pharmacy at the University of Kentucky
bound metal that is presumed to be either magnesium or manganese (Ke and Wang
2007b
; Sung et al.
2003
; Xu et al.
2000
). The exact complement of metal ions that
occupy this binuclear site has not been defined for any mammalian PDE since
catalysis in various PDEs is preferentially supported by different metals including
magnesium, manganese, cobalt, or zinc.
A zinc atom is clearly defined in most of the X-ray crystal structures of PDE
catalytic domains and is present even when crystals are formed in the presence of
chelators (Ke and Wang
2007b
; Xu et al.
2000
), but the metal ion occupying the
second site has not been identified for any PDE. The X-ray structure of the refolded
PDE3 catalytic domain is the only structure of a wild-type PDE protein determined
thus far that lacks a zinc ion (Scapin et al.
2004
). By direct chemical analysis, PDE6
has been shown to contain 3-4 zincs per dimer. Zinc is also critical for PDE6
catalytic function (He et al.
2000
), but magnesium also stimulates PDE6 activity.
Likewise, PDE5 binds ~6 zincs per dimer, and zinc at submicromolar levels support
catalytic function, but manganese, cobalt, and magnesium at higher concentrations
also support catalysis (Francis et al.
1994
). The precise role of the respective metal
ions in the catalytic function of each PDE is still not well understood. To exploit the
effect of an inhibitor to interfere with the role of these metal ions in PDE functions,
it will be critical to better define the particular metal complement that is important
for catalytic function of a PDE isoform.
The volume of the PDE catalytic sites has been estimated to be ~330-450
˚
3
,
and several structures of isolated catalytic domains in complex with either the