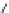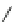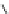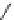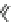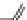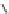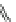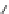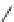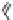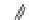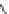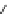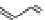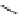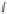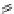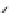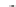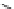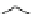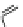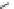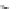Biology Reference
In-Depth Information
PROTOTYPE
ADVANCED PDE INHIBITORS
H
F
H
H
F
N
NN
N
N
O
N
O
O
O
O
NH
2
N
Amrinone
Milrinone
Zardaverine
N
F
NH
O
O
O
OCl
OCl
F
O
O
OH
N
H
N
N
H
N
O
O
O
O
O
Cl
Cl
Rolipram
Cilomilast
Piclamilast
Roflumilast
N
N
H
O
O
O
H
N
N
N
O
O
O
O
O
O
NH
HN
Papaverine
S
O
Benafentrine
O
Tolafentrine
O
O
H
N
HN
N
HN
O
O
O
N
N
S
N
N
N
N
N
O
Zaprinast
Sildenafil
Fig. 1 Chemical structures of prototypic and advanced PDE inhibitors
contrary, these compounds served as lead structures for chemical optimisation (for
some examples see Fig.
1
) and were useful tools for PDE research especially for
evaluating the relevant PDE functions in intact cells and tissues.
3.3 Six Lessons About PDE Inhibitor Function in Isolated Organs
3.3.1 Potentiation of Adenylate and Guanylate Cyclase Agonists
Isolated organs such as aorta, pulmonary artery, intestinal segments from ileum and
perfused heart had been established and used for characterisation of mediators,
contractile agonists and antagonists since the beginning of the last century. In
vascular preparations, papaverine differed from the weaker theophylline because
it was a very potent relaxing agent by itself and was therefore called a “direct vas-
orelaxant” (Poech and Kukovetz
1971
). Theophylline, however, seemed to be more
efficacious in perfused heart preparations, isolated papillary muscle or isolated atria
(Rall and West 1963). Both compounds relaxed the various preparations (1) without