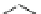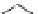Biology Reference
In-Depth Information
H
N
O
O
O
N
N
N
N
N
O
N
N
O
N
Cilostazol
pentoxifylline
H
N
O
O
O
Br
HN
N
N
H
N
O
CI
N
H
OH
O
N
HCI
K-134 (OPC-33509)
NM-702 (NT-702)
Fig. 4 Chemical structures of PDE inhibitors that have been evaluated for the treatment of
intermittent claudication
Table 1 IC
50
(
m
M) of PDE inhibitors developed for use in treatment of intermittent claudication
PDE
1
2
3A
3B
4
5
Cilostazol
>
100 (Sudo
et al.
2000
)
45.2 (Sudo
et al.
2000
)
0.2 (Sudo et al.
2000
)
0.38 (Sudo
et al.
2000
)
88.0 (Sudo
et al.
2000
)
4.4 (Sudo et al.
2000
)
Pentoxifylline
a
>
100
a
>
100
a
30 ~ 100
a
44
a
NT-702
(NM-702)
3,340
(Ishiwata
et al.
2007
)
137 (Ishiwata
et al.
2007
)
0.179
(Ishiwata
et al.
2007
)
0.260
(Ishiwata
et al.
2007
)
1,240
(Ishiwata
et al.
2007
)
87.2 (Ishiwata
et al.
2007
)
K-134 (OPC-
33509)
>
300 (Sudo
et al.
2000
)
>
300 (Sudo
et al.
2000
)
0.10 (Sudo
et al.
2000
)
0.28 (Sudo
et al.
2000
)
>
300 (Sudo
12.1 (Sudo
et al.
2000
)
et al.
2000
)
Values were obtained using recombinant human PDEs expressed in Sf9 insect cells. For details,
please see corresponding references. Also, isoform-specific values for particular PDE families are not
listed, except for PDE3. In general, these inhibitors do not discriminate between different isoforms
a
Values for pentoxifylline were obtained from Caliper Life Sciences website
http://www.caliperls.
com/assets/022/8270.pdf
(last access on Dec 2009)
uptake, but cilostazol is a much more potent inhibitor [IC
50
: 3-5
m
M (Liu et al.
2000
) vs.
100
m
M for pentoxifylline (de la Cruz et al.
1993
)].
>
4.1 The Success Story of Cilostazol
4.1.1 Clinical Studies Leading to the Approval of Cilostazol
for Relief of IC Symptoms
In the early 1990s, Otsuka began to evaluate the effect of cilostazol to increase
walking distance in IC patients. Results from clinical trials in the USA in patients
with moderate-to-severe IC demonstrated that cilostazol significantly increased