Biology Reference
In-Depth Information
C-terminal helix is short (~15 amino acids) and likely only allows the C-terminal
helix to interact intramolecularly with the adjacent active site of the same monomer
in all of these structures.
The UCR2 and C-terminal regulatory domain structures suggest a “dual-gating”
mechanism regulating PDE4 hydrolysis of cAMP (Houslay and Adams
2010
;
Burgin et al.
2010
). In this model, UCR2 and the C-terminal gating helix indepen-
dently regulate PDE enzymatic activity (Fig.
3
). The C-terminal helix likely acts
intramolecularly to regulate the adjacent active site, while UCR2 may act intermo-
lecularly. We propose that the kinetic behavior of atypical PDE4 inhibitors indi-
cates that the two active sites are negatively cooperative such that when the UCR2
from one of the monomers in the dimer closes over the active site of the other
monomer in the dimer, this engenders a greatly reduced affinity of the other UCR2
for interacting with the other active site in the proposed dimeric unit. Indeed, this
can be best explained by a model in which biological isoforms of PDE4 are
functional dimers(Lee et al.
2002
; Richter and Conti
2002
). Closing UCR2 creates
an asymmetric dimer in which one active site is fully inhibited and the opposite
active site has reduced catalytic activity (Burgin et al.
2010
). In this model, we
have suggested that UCR2 likely acts intermolecularly such that UCR2 from one
monomer closes across the active site of the opposite monomer, Fig.
3
(Burgin et al.
2010
). This highlights the functional importance of PDE4 dimerization with respect
to regulation of activity. Indeed, accessory proteins that interact with the PDE4
monomer and thereby prevent dimerization may prevent UCR2 gating.
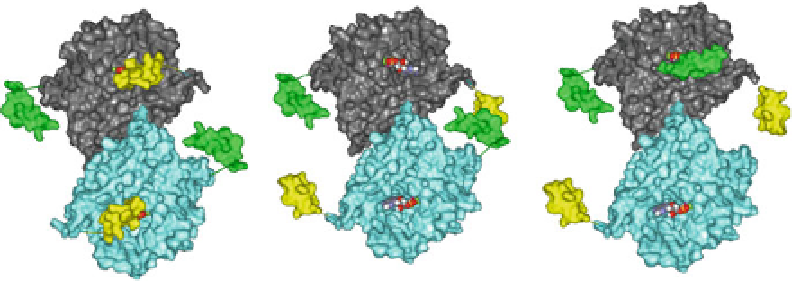
cAMP
Symmetric Closed Conformer
Active sites are independent
Inhibitors close C-terminal helices
across both active sites
Inhibitors can be isoform selective
PDE activity is completely
inhibited
Symmetric Open Conformer
Active sites are independent
Inhibitors bind in active site
competitively with cAMP
Inhibitors are non-selective
PDE activity is completely
inhibited
Asymmetric Closed Conformer
Active sites are negatively cooperative
Allosteric modulators close UCR2 from one
monomer across the active site of the
opposite monomer, but cannot close
UCR2 across both active sites
Modulators can be isoform selective
PDE activity is only PARTIALLY inhibited
Fig. 3 PDE4 conformers that may be exploited by therapeutic agents. In the model, the PDE4
dimer can exist as a symmetric closed conformer with the C-terminal helix capping both active
sites, it can exist as an open symmetric monomer with both active sites uncapped, and it can exist as
an asymmetric dimer with UCR2 capping one active site, while the other active site remains open.
The catalytic domains of the PDE4 monomers are surface rendered in
blue
or
magenta
. The
C-terminal gating helix is rendered in
yellow
, while the UCR2-gating helix is rendered in
green
or
red