Biology Reference
In-Depth Information
Beavo
2006
; Conti and Beavo
2007
) require more severe disabling of cAMP
signaling conferred by the loss of the Gpa2 G
a
to produce a tight 5FOA
S
pheno-
type. Loss of Gpa2 is not sufficient to confer 5FOA
S
growth in strains expressing
the cAMP-specific IBMX-insensitive PDE8A enzyme (Bender and Beavo
2006
;
Conti and Beavo
2007
), suggesting very low activity of this enzyme when
expressed in
S. pombe
(in these strains, the full-length mammalian genes are
expressed from the yeast PDE gene promoter at its genomic locus). The 5FOA
S
strains are suitable for HTSs for PDE inhibitors that elevate cAMP levels and thus
repress
fbp1-ura4
transcription to allow 5FOA
R
growth (Fig.
2
). However, this
approach is not amenable for screening strains expressing PDEs such as PDE8A
whose activity is insufficient to confer 5FOA
S
growth in these mutant backgrounds.
HTSs were optimized using a 384 well format. Assay development involves
optimizing growth conditions prior to screening to prevent
ura4
reporter expression
(thus PDE inhibitors maintain, rather than establish, repression of the reporter) and
initial cell density. A 48-h incubation period is required for actively growing strains
to reach saturation, allowing the greatest contrast in optical density between
vehicle-treated cultures (OD
600
¼
0.05-0.2) and PDE inhibitor-treated or cAMP-
treated cultures that reach a saturated cell density (OD
600
¼
1.2-1.3). Optimized
assays generally produce Z-factors of 0.7-0.9, indicative of highly robust screens.
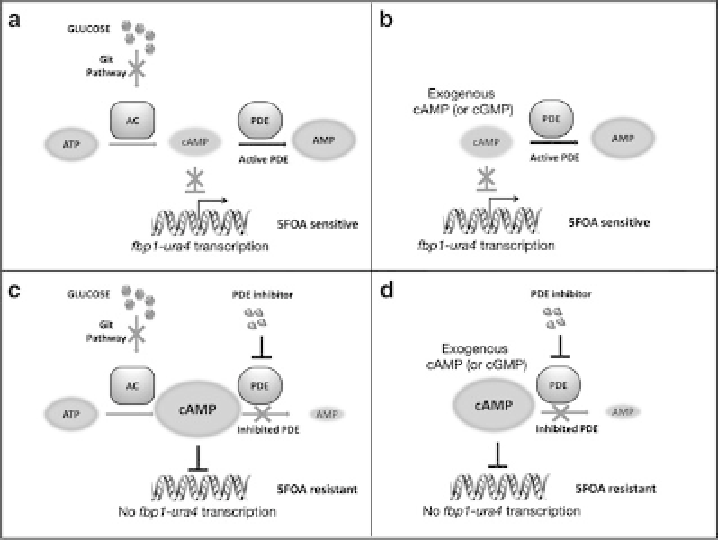
Fig. 2 First, second and third generation 5FOA-growth screens. (a) 5FOA
S
growth in a first
generation screening strain is due to a defective glucose-sensing Git pathway. (b) 5FOA
S
growth
in a second or third generation screening strain is due to PDE degradation of exogenously added
cAMP or cGMP. (c, d) PDE inhibition elevates intracellular cAMP or cGMP levels to repress
fbp1-ura4
transcription and confer 5FOA
R
growth