Biology Reference
In-Depth Information
On the basis of this profile, it is obvious again that the active metabolite is
considered to largely account for the clinical efficacy of roflumilast documented in
COPD patients (Calverley et al.
2009
; Fabbri et al.
2009
).
Roflumilast is unique in its class with both metabolite and parent drug contribut-
ing to PDE4 inhibition. The resulting overall pharmacodynamic effect is main-
tained over the dosing interval, irrespective of factors influencing the metabolic
conversion of roflumilast to its N-oxide. This adds to the predictability of efficacy
and safety in COPD patients by avoiding therapeutic failure or adverse drug
reactions in case of concomitant medication or comorbidities, because the effect
of increased or decreased metabolism will be outbalanced. The parallel metabolic
pathways of roflumilast by CYP 3A4 and 1A2 additionally compensate effects of
coadministration of roflumilast and enzyme inhibitors. As confirmed by drug-drug
interaction studies, roflumilast reveals a common interaction profile on the basis of
its CYP 3A4 and 1A2 metabolism. When given together with enoxacin, cimetidin,
fluvoxamin, and rifampicin, the efficacy and safety needs to be clinically followed.
The striking difference and progress in the pharmacokinetic profiles from the
early rolipram to roflumilast is schematically illustrated by comparing plasma
levels over time following repeated administration of their clinical doses in humans
(Fig.
6
).
a
b
Rolipram
3
Roflumilast N-oxide
15
2
10
1
5
0
0
02468 0 2 4 6 8 0 2 4
0 2 4 6 8 1012141618202224
Time (h)
Time (h)
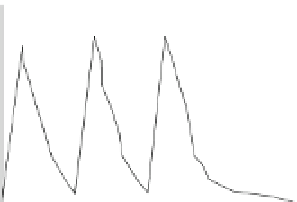
Fig. 6 Plasma levels over time (unbound to plasma protein) following repeated oral administra-
tion of roflumilast (500
m
g, once daily) (a) or rolipram (750
m
g, three times daily) (b). In (a),
plasma levels for roflumilast N-oxide are shown, given that it accounts for
>
90% of overall PDE4
inhibition. In (b), plasma levels are adapted from Krause et al.
1989
considering plasma protein
binding of 85%
For roflumilast N-oxide, plasma levels unbound to protein remain in a rather
narrow range over an entire 24 h dosing interval. In contrast, rolipram plasma levels
are fluctuating by more than 15-fold between trough and peak concentrations.
While evaluating these profiles, it is intriguing that, in general, unfolding anti-
inflammatory effects of PDE4 inhibitors in the clinics likely require a rather
constant level of PDE4 inhibition over a dosing interval. Side effects (such as
nausea) are perceived as occurring rapidly, yet being rather transient in nature. One