Chemistry Reference
In-Depth Information
(a)
(b)
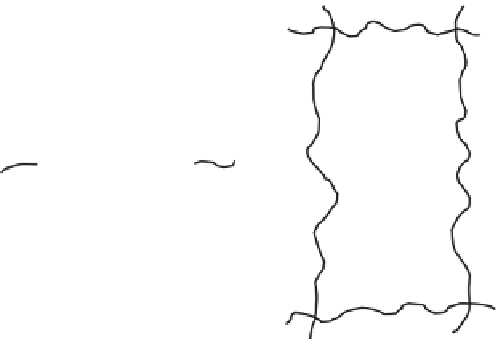
Fi g u re 7.11:
Two end-linking techniques for preparing networks with known numbers and lengths of
dangling chains. In part
a
, the dangling chains are produced by having more chain ends
than complementary groups on the end-linking agent, and in part
b
by preparing some
chains to have reactive groups on only one of the two chain ends.
More definitive come from a series of model networks prepared by
end linking vinyl-terminated PDMS chains.
171,
173
The tetrafunctional
end-linking agent was varied at levels below stoichiometric balance be-
tween its active hydrogen atoms and the chains' terminal vinyl groups.
The ultimate properties of these networks, with known numbers of
dangling ends, were then compared with those obtained on networks
with negligible numbers of these irregularities.
171
Values of
f
*
r
of the
networks containing the dangling ends was lower than that of the
more perfect networks, with the largest differences occurring at high
proportions of dangling ends (low 2
C
1
), as expected.
171
These results,
shown schematically in figure 7.12, thus confirm the results shown in
figure 7.10. Maximum extensibility shows a similar dependence, as
expected.
7.2.6 Interpenetrating Networks
If two types of chains have different end groups, then it is possible to end
link them simultaneously into two networks that
interpenetrate
one an-
other.
174
Such a network (figure 7.13) could, for example, be made by re-
acting hydroxyl-terminated PDMS chains with tetraethoxysilane (in a
condensation reaction), while reacting vinyl-terminated PDMS chains